HPV Vaccination during the COVID-19 Pandemic in Italy: Opportunity Loss or Incremental Cost
- PMID: 35891297
- PMCID: PMC9322500
- DOI: 10.3390/vaccines10071133
HPV Vaccination during the COVID-19 Pandemic in Italy: Opportunity Loss or Incremental Cost
Abstract
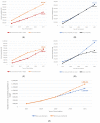
Objectives: Italy was the first European country to introduce universal vaccination of adolescents, for both males and females, against Human Papilloma Virus (HPV) starting in 2017 with the NIP 2017-2019's release. However, vaccine coverage rates (VCRs) among adolescents have shown a precarious take-off since the NIP's release, and this situation worsened due to the impact of the COVID-19 pandemic in 2020. The aim of this work is to estimate the epidemiological and economic impact of drops in VCRs due to the pandemic on those generations that missed the vaccination appointment and to discuss alternative scenarios in light of the national data.
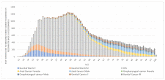
Methods: Through an analysis of the official ministerial HPV vaccination reports, a model was developed to estimate the number of 12-year-old males and females who were not vaccinated against HPV during the period 2017-2021. Based on previously published models that estimate the incidence and the economic impact of HPV-related diseases in Italy, a new model was developed to estimate the impact of the aggregated HPV VCRs achieved in Italy between 2017 and 2021.
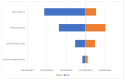
Results: Overall, in 2021, 723,375 girls and 1,011,906 boys born between 2005 and 2009 were not vaccinated against HPV in Italy (42% and 52% of these cohorts, respectively). As compared with the 95% target provided by the Italian NIP, between 505,000 and 634,000 girls will not be protected against a large number of HPV-related diseases. For boys, the number of the unvaccinated population compared to the applicable target is over 615,000 in the 'best case scenario' and over 749,000 in the 'worst case scenario'. Overall, between 1.1 and 1.3 million young adolescents born between 2005 and 2009 will not be protected against HPV-related diseases over their lifetime with expected lifetime costs of non-vaccination that will be over EUR 905 million. If the 95% optimal VCRs were achieved, the model estimates a cost reduction equal to EUR 529 million, the net of the costs incurred to implement the vaccination program.
Conclusion: Suboptimal vaccination coverage represents a missed opportunity, not only because of the increased burden of HPV-related diseases, but also in terms of economic loss. Thus, reaching national HPV immunization goals is a public health priority.
Keywords: COVID-19; HPV; HTA; RWD; incremental cost; vaccination strategies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials

