A Recombinant Chimeric Protein-Based Vaccine Containing T-Cell Epitopes from Amastigote Proteins and Combined with Distinct Adjuvants, Induces Immunogenicity and Protection against Leishmania infantum Infection
- PMID: 35891310
- PMCID: PMC9317424
- DOI: 10.3390/vaccines10071146
A Recombinant Chimeric Protein-Based Vaccine Containing T-Cell Epitopes from Amastigote Proteins and Combined with Distinct Adjuvants, Induces Immunogenicity and Protection against Leishmania infantum Infection
Abstract
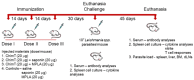
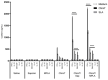
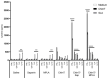
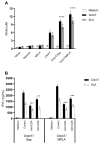
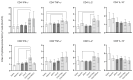
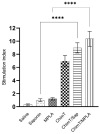
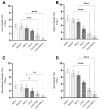
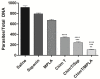
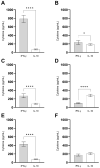
Currently, there is no licensed vaccine to protect against human visceral leishmaniasis (VL), a potentially fatal disease caused by infection with Leishmania parasites. In the current study, a recombinant chimeric protein ChimT was developed based on T-cell epitopes identified from the immunogenic Leishmania amastigote proteins LiHyp1, LiHyV, LiHyC and LiHyG. ChimT was associated with the adjuvants saponin (Sap) or monophosphoryl lipid A (MPLA) and used to immunize mice, and their immunogenicity and protective efficacy were evaluated. Both ChimT/Sap and ChimT/MPLA induced the development of a specific Th1-type immune response, with significantly high levels of IFN-γ, IL-2, IL-12, TNF-α and GM-CSF cytokines produced by CD4+ and CD8+ T cell subtypes (p < 0.05), with correspondingly low production of anti-leishmanial IL-4 and IL-10 cytokines. Significantly increased (p < 0.05) levels of nitrite, a proxy for nitric oxide, and IFN-γ expression (p < 0.05) were detected in stimulated spleen cell cultures from immunized and infected mice, as was significant production of parasite-specific IgG2a isotype antibodies. Significant reductions in the parasite load in the internal organs of the immunized and infected mice (p < 0.05) were quantified with a limiting dilution technique and quantitative PCR and correlated with the immunological findings. ChimT/MPLA showed marginally superior immunogenicity than ChimT/Sap, and although this was not statistically significant (p > 0.05), ChimT/MPLA was preferred since ChimT/Sap induced transient edema in the inoculation site. ChimT also induced high IFN-γ and low IL-10 levels from human PBMCs isolated from healthy individuals and from VL-treated patients. In conclusion, the experimental T-cell multi-epitope amastigote stage Leishmania vaccine administered with adjuvants appears to be a promising vaccine candidate to protect against VL.
Keywords: T-cell epitopes; adjuvants; immune response; polypeptide-based protein; vaccine; visceral leishmaniasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Efficacy of an Immunotherapy Combining Immunogenic Chimeric Protein Plus Adjuvant and Amphotericin B against Murine Visceral Leishmaniasis.Biology (Basel). 2023 Jun 13;12(6):851. doi: 10.3390/biology12060851. Biology (Basel). 2023. PMID: 37372136 Free PMC article.
-
Recombinant endonuclease III protein from Leishmania infantum associated with Th1-type adjuvants is immunogenic and induces protection against visceral leishmaniasis.Mol Immunol. 2023 Mar;155:79-90. doi: 10.1016/j.molimm.2023.01.011. Epub 2023 Jan 31. Mol Immunol. 2023. PMID: 36731193
-
A Leishmania amastigote-specific hypothetical protein evaluated as recombinant protein plus Th1 adjuvant or DNA plasmid-based vaccine to protect against visceral leishmaniasis.Cell Immunol. 2020 Oct;356:104194. doi: 10.1016/j.cellimm.2020.104194. Epub 2020 Aug 13. Cell Immunol. 2020. PMID: 32827943
-
A new chimeric protein composed by T-cell epitopes from peroxidoxin and pyridoxal kinase proteins is protective against visceral leishmaniasis.Cell Immunol. 2025 May-Jun;411-412:104949. doi: 10.1016/j.cellimm.2025.104949. Epub 2025 Apr 4. Cell Immunol. 2025. PMID: 40198961
-
Generation of growth arrested Leishmania amastigotes: a tool to develop live attenuated vaccine candidates against visceral leishmaniasis.Vaccine. 2014 Jun 30;32(31):3895-901. doi: 10.1016/j.vaccine.2014.05.009. Epub 2014 May 14. Vaccine. 2014. PMID: 24837513 Review.
Cited by
-
Blue light-emitting diode phototherapy presents in vitro efficacy against distinct Leishmania species and is therapeutic against tegumentary leishmaniasis in BALB/c mice.Front Immunol. 2025 May 5;16:1554051. doi: 10.3389/fimmu.2025.1554051. eCollection 2025. Front Immunol. 2025. PMID: 40391226 Free PMC article.
-
Immunogenicity and protective efficacy of tuzin protein as a vaccine candidate in Leishmania donovani-infected BALB/c mice.Front Immunol. 2024 Jan 11;14:1294397. doi: 10.3389/fimmu.2023.1294397. eCollection 2023. Front Immunol. 2024. PMID: 38274802 Free PMC article.
-
Evaluation of the humoral and mucosal immune response of a multiepitope vaccine against COVID-19 in pigs.Front Immunol. 2023 Dec 20;14:1276950. doi: 10.3389/fimmu.2023.1276950. eCollection 2023. Front Immunol. 2023. PMID: 38179057 Free PMC article.
-
Vaccine Designing Technology against Leishmaniasis: Current Challenges and Implication.Curr Drug Discov Technol. 2025;22(2):e240524230315. doi: 10.2174/0115701638291767240513113400. Curr Drug Discov Technol. 2025. PMID: 38798212 Review.
-
Design and development of highly conserved, HLA-promiscuous T cell multiepitope vaccines against human visceral leishmaniasis.Front Immunol. 2025 Mar 31;16:1540537. doi: 10.3389/fimmu.2025.1540537. eCollection 2025. Front Immunol. 2025. PMID: 40230841 Free PMC article.
References
-
- Organisation W.H. Leishmaniasis. [(accessed on 1 July 2022)]. Available online: http://www.who.int/topics/leishmaniasis/en/
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

