The Impact of Early Antiretroviral Treatment (ART) for HIV on the Sensitivity of the Latest Generation of Blood Screening and Point of Care Assays
- PMID: 35891406
- PMCID: PMC9324335
- DOI: 10.3390/v14071426
The Impact of Early Antiretroviral Treatment (ART) for HIV on the Sensitivity of the Latest Generation of Blood Screening and Point of Care Assays
Abstract
Introduction: Rapid initiation of antiretroviral therapy (ART) in early HIV infection is important to limit seeding of the viral reservoir. A number of studies have shown that if ART is commenced prior to seroconversion, the seroconversion may, or may not, occur. We aimed to assess whether seroreversion or no seroconversion occurs using samples collected during an early treatment study in South Africa.
Methods: We tested 10 longitudinal samples collected over three years from 70 blood donors who initiated ART after detection of acute or early HIV infection during donation screening on fourth- and fifth-generation HIV antibody and RNA assays, and three point of care (POC) rapid tests. Donors were allocated to three treatment groups: (1) very early, (2) early, and (3) later. Longitudinal samples were grouped into time bins post-treatment initiation.
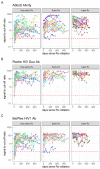
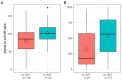
Results: On all three high-throughput HIV antibody assays, no clear pattern of declining signal intensity was observed over time after ART initiation in any of the treatment initiation groups and 100% detection was obtained. The Abbott Determine POC assay showed 100% detection at all time points with no seroreversion. However, the Abbott ABON HIV1 and OraSure OraQuick POC assays showed lower proportions of detection in all time bins in the very early treated group, ranging from 50.0% (95% CI: 26.8-73.2%) to 83.1% (95% CI: 64.2-93.0%), and moderate detection rates in the early and later-treated groups.
Conclusion: While our findings are generally reassuring for HIV detection when high-throughput serological screening assays are used, POC assays may have lower sensitivity for detection of HIV infection after early treatment. Findings are relevant for blood safety and other settings where POC assays are used.
Keywords: HIV; antiretroviral therapy; point of care testing; serological screening.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ananworanich J., Schuetz A., Vandergeeten C., Sereti I., de Souza M., Rerknimitr R., Dewar R., Marovich M., van Griensven F., Sekaly R., et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS ONE. 2012;7:e33948. doi: 10.1371/journal.pone.0033948. - DOI - PMC - PubMed
-
- Ananworanich J., Sacdalan C.P., Pinyakorn S., Chomont N., Souzade M., Luekasemsuk T., Schuetz A., Krebs S.J., Dewar R., Jagodzinski L., et al. Virological and immunological characteristics of HIV-infected individuals at the earliest stage of infection. J. Virus Erad. 2016;2:43–48. doi: 10.1016/S2055-6640(20)30688-9. - DOI - PMC - PubMed
-
- Schuetz A., Deleage C., Sereti I., Rerknimitr R., Phanuphak N., Phuang-Ngern Y., Estes J.D., Sandler N.G., Sukhumvittaya S., Marovich M., et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog. 2014;10:e1004543. doi: 10.1371/journal.ppat.1004543. - DOI - PMC - PubMed
-
- Henrich T.J., Hatano H., Bacon O., Hogan L.E., Rutishauser R., Hill A., Kearney M.F., Anderson E.M., Buchbinder S.P., Cohen S.E., et al. HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study. PLoS Med. 2017;14:e1002417. doi: 10.1371/journal.pmed.1002417. - DOI - PMC - PubMed
-
- de Souza M.S., Pinyakorn S., Akapirat S., Pattanachaiwit S., Fletcher J.L., Chomchey N., Kroon E.D., Ubolyam S., Michael N.L., Robb M.L., et al. Initiation of Antiretroviral Therapy During Acute HIV-1 Infection Leads to a High Rate of Nonreactive HIV Serology. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016;63:555–561. doi: 10.1093/cid/ciw365. - DOI - PubMed

