Polymorphic Forms of Human Cytomegalovirus Glycoprotein O Protect against Neutralization of Fibroblast Entry by Antibodies Targeting Epitopes Defined by Glycoproteins H and L
- PMID: 35891489
- PMCID: PMC9323020
- DOI: 10.3390/v14071508
Polymorphic Forms of Human Cytomegalovirus Glycoprotein O Protect against Neutralization of Fibroblast Entry by Antibodies Targeting Epitopes Defined by Glycoproteins H and L
Abstract
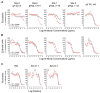
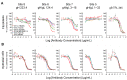
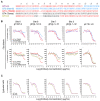
Human cytomegalovirus (CMV) utilizes different glycoproteins to enter into fibroblast and epithelial cells. A trimer of glycoproteins H, L, and O (gH/gL/gO) is required for entry into all cells, whereas a pentamer of gH/gL/UL128/UL130/UL131A is selectively required for infection of epithelial, endothelial, and some myeloid-lineage cells, but not of fibroblasts. Both complexes are of considerable interest for vaccine and immunotherapeutic development but present a conundrum: gH/gL-specific antibodies have moderate potency yet neutralize CMV entry into all cell types, whereas pentamer-specific antibodies are more potent but do not block fibroblast infection. Which cell types and neutralizing activities are important for protective efficacy in vivo remain unclear. Here, we present evidence that certain CMV strains have evolved polymorphisms in gO to evade trimer-specific neutralizing antibodies. Using luciferase-tagged variants of strain TB40/E in which the native gO is replaced by gOs from other strains, we tested the effects of gO polymorphisms on neutralization by monoclonal antibodies (mAbs) targeting four independent epitopes in gH/gL that are common to both trimer and pentamer. Neutralization of fibroblast entry by three mAbs displayed a range of potencies that depended on the gO type, a fourth mAb failed to neutralize fibroblast entry regardless of the gO type, while neutralization of epithelial cell entry by all four mAbs was potent and independent of the gO type. Thus, specific polymorphisms in gO protect the virus from mAb neutralization in the context of fibroblast but not epithelial cell entry. No influence of gO type was observed for protection against CMV hyperimmune globulin or CMV-seropositive human sera, suggesting that antibodies targeting protected gH/gL epitopes represent a minority of the polyclonal neutralizing repertoire induced by natural infection.
Keywords: cytomegalovirus; glycoprotein O; hyperimmune globulin; immune evasion; neutralizing antibody; polymorphism.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures