SARS-CoV-2 Omicron Induces Enhanced Mucosal Interferon Response Compared to other Variants of Concern, Associated with Restricted Replication in Human Lung Tissues
- PMID: 35891570
- PMCID: PMC9318963
- DOI: 10.3390/v14071583
SARS-CoV-2 Omicron Induces Enhanced Mucosal Interferon Response Compared to other Variants of Concern, Associated with Restricted Replication in Human Lung Tissues
Abstract
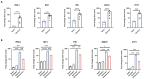
SARS-CoV-2 Omicron variant has been characterized by decreased clinical severity, raising the question of whether early variant-specific interactions within the mucosal surfaces of the respiratory tract could mediate its attenuated pathogenicity. Here, we employed ex vivo infection of native human nasal and lung tissues to investigate the local-mucosal susceptibility and innate immune response to Omicron compared to Delta and earlier SARS-CoV-2 variants of concern (VOC). We show that the replication of Omicron in lung tissues is highly restricted compared to other VOC, whereas it remains relatively unchanged in nasal tissues. Mechanistically, Omicron induced a much stronger antiviral interferon response in infected tissues compared to Delta and earlier VOC-a difference, which was most striking in the lung tissues, where the innate immune response to all other SARS-CoV-2 VOC was blunted. Notably, blocking the innate immune signaling restored Omicron replication in the lung tissues. Our data provide new insights to the reduced lung involvement and clinical severity of Omicron.
Keywords: COVID-19; Omicron; SARS-CoV-2; interferon response; lung organ culture; nasal organ culture; organ culture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

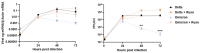
References
-
- Wolter N., Jassat W., Walaza S., Welch R., Moultrie H., Groome M., Amoako D.G., Everatt J., Bhiman J.N., Scheepers C., et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: A data linkage study. Lancet. 2022;399:437–446. doi: 10.1016/S0140-6736(22)00017-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

