Growth parameters and responses of green algae across a gradient of phototrophic, mixotrophic and heterotrophic conditions
- PMID: 35891646
- PMCID: PMC9308967
- DOI: 10.7717/peerj.13776
Growth parameters and responses of green algae across a gradient of phototrophic, mixotrophic and heterotrophic conditions
Abstract
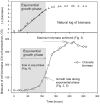
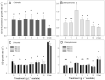
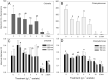
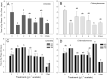
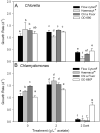
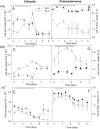
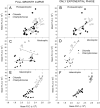
Many studies have shown that algal growth is enhanced by organic carbon and algal mixotrophy is relevant for physiology and commercial cultivation. Most studies have tested only a single organic carbon concentration and report different growth parameters which hampers comparisons and improvements to algal cultivation methodology. This study compared growth of green algae Chlorella vulgaris and Chlamydomonas reinhardtii across a gradient of photoautotrophic-mixotrophic-heterotrophic culture conditions, with five acetate concentrations. Culture growth rates and biomass achieved were compared using different methods of biomass estimation. Both species grew faster and produced the most biomass when supplied with moderate acetate concentrations (1-4 g L-1), but light was required to optimize growth rates, biomass yield, cell size and cell chlorophyll content. Higher acetate concentration (10 g L-1) inhibited algal production. The choice of growth parameter and method to estimate biomass (optical density (OD), chlorophyll a fluorescence, flow cytometry, cell counts) affected apparent responses to organic carbon, but use of OD at 600, 680 or 750 nm was consistent. There were apparent trade-offs among exponential growth rate, maximum biomass, and culture time spent in exponential phase. Different cell responses over 1-10 g L-1 acetate highlight profound physiological acclimation across a gradient of mixotrophy. In both species, cell size vs cell chlorophyll relationships were more constrained in photoautotrophic and heterotrophic cultures, but under mixotrophy, and outside exponential growth phase, these relationships were more variable. This study provides insights into algal physiological responses to mixotrophy but also has practical implications for choosing parameters for monitoring commercial algal cultivation.
Keywords: Acetate; Algal cultures; Carrying capacity; Chlorophyll a fluorescence; Exponential growth; Flow cytometry; Optical density; Photosynthesis; Physiology.
© 2022 Young et al.
Conflict of interest statement
John A. Berges is an Academic Editor for PeerJ.
Figures
References
-
- Abiusi F, Moniño Fernández P, Canziani S, Janssen M, Wijffels RH, Barbosa M. Mixotrophic cultivation of Galdieria sulphuraria for C-phycocyanin and protein production. Algal Research. 2022;61:102603. doi: 10.1016/j.algal.2021.102603. - DOI
-
- Andersen T, Faeerovig PJ, Hessen DO. Growth rate versus biomass accumulation: different roles of food quality and quantity for consumers. Limnology and Oceanography. 2007;52:2128–2134. doi: 10.4319/lo.2007.52.5.2128. - DOI
-
- Anderson RA, Berges JA, Harrison PJ, Watanabe MM. Appendix A—recipes for freshwater and seawater media. In: Andersen RA, editor. Algal Culturing Techniques. Cambridge: Elsevier Academic Press; 2005. pp. 429–538.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

