Use of erythropoiesis-stimulating agents in children with chronic kidney disease: a systematic review
- PMID: 35892014
- PMCID: PMC9308099
- DOI: 10.1093/ckj/sfac058
Use of erythropoiesis-stimulating agents in children with chronic kidney disease: a systematic review
Abstract
Background: Erythropoiesis-stimulating agents (ESAs) revolutionized the management of anaemia in chronic kidney disease (CKD) when introduced in the late 1980s. A range of ESA types, preparations and administration modalities now exist, with newer agents requiring less frequent administration. Although systematic reviews and meta-analyses have been published in adults, no systematic review has been conducted investigating ESAs in children.
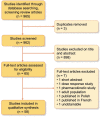
Methods: The Preferred Reporting Items for Systematic Reviews and Meta-analyses statement for the conduct of systematic reviews was used. All available literature on outcomes relating to ESAs in children with CKD was sought. A search of the MEDLINE, CINAHL and Embase databases was conducted by two independent reviewers. Inclusion criteria were published trials in English, children with chronic and end-stage kidney disease and use of any ESA studied against any outcome measure. An assessment of risk of bias was carried out in all included randomized trials using the criteria from the Cochrane Handbook for Systematic Reviews of Interventions. Two tables were used for data extraction for randomized and observational studies. Study type, participants, inclusion criteria, case characteristics, follow-up duration, ESA type and dosage, interventions and outcomes were extracted by one author.
Results: Of 965 identified articles, 58 were included covering 54 cohorts. Six were randomized trials and 48 were observational studies. A total of 38 studies assessed the efficacy of recombinant human erythropoietin (rHuEPO), 11 of darbepoetin alpha (DA) and 3 of continuous erythropoietin receptor activator (CERA), with 6 studies appraising secondary outcome measures exclusively. Recruitment to studies was a consistent challenge. The most common adverse effect was hypertension, although confounding effects often limited direct correlation. Two large cohort studies demonstrated a greater hazard of death independently associated with high ESA dose. Secondary outcome measures included quality of life measures, growth and nutrition, exercise capacity, injection site pain, cardiovascular function, intelligent quotient, evoked potentials and platelet function.
Conclusions: All ESA preparations and modes of administration were efficacious, with evidence of harm at higher doses. Evidence supports individualizing treatments, with strong consideration given to alternate treatments in patients who appear resistant to ESA therapy. Further research should focus on randomized trials comparing the efficacy of different preparations, treatment options in apparently ESA-resistant cohorts and clarification of meaningful secondary outcomes to consolidate patient-relevant indices.
Keywords: ESA; chronic renal failure; haemoglobin; paediatrics; systematic review.
© The Author(s) 2022. Published by Oxford University Press on behalf of the ERA.
Figures
Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
Types of erythropoiesis-stimulating agents and risk of end-stage kidney disease and death in patients with non-dialysis chronic kidney disease.Nephrol Dial Transplant. 2021 Jan 25;36(2):267-274. doi: 10.1093/ndt/gfaa088. Nephrol Dial Transplant. 2021. PMID: 32829405
-
Comparative efficacy and safety in ESA biosimilars vs. originators in adults with chronic kidney disease: a systematic review and meta-analysis.J Nephrol. 2018 Jun;31(3):321-332. doi: 10.1007/s40620-017-0419-5. Epub 2017 Jun 23. J Nephrol. 2018. PMID: 28646375
-
Efficacy and Cardiovascular Adverse Effects of Erythropoiesis Stimulating Agents in the Treatment of Cancer-Related Anemia: A Systematic Review of Randomized Controlled Trials.Cureus. 2021 Sep 8;13(9):e17835. doi: 10.7759/cureus.17835. eCollection 2021 Sep. Cureus. 2021. PMID: 34527499 Free PMC article. Review.
Cited by
-
Evaluation of recombinant human erythropoietin as a promoter of bone healing in cats with femoral fractures.Open Vet J. 2024 Apr;14(4):1012-1018. doi: 10.5455/OVJ.2024.v14.i4.8. Epub 2024 Apr 30. Open Vet J. 2024. PMID: 38808286 Free PMC article.
-
ENDOCRINE AND GROWTH ABNORMALITIES IN CHILDREN WITH KIDNEY FAILURE ON MAINTENANCE HEMODIALYSIS - EXPERIENCE OF A SINGLE CENTER FROM WESTERN ROMANIA.Acta Endocrinol (Buchar). 2024 Jul-Sep;20(3):393-400. doi: 10.4183/aeb.2024.393. Epub 2025 May 23. Acta Endocrinol (Buchar). 2024. PMID: 40530097 Free PMC article.
-
Comparative efficacy of epoetin alfa vs. darbepoetin in children with chronic kidney disease: a systematic review, meta-analysis and cost-effectiveness analysis.J Nephrol. 2025 May 11. doi: 10.1007/s40620-025-02303-8. Online ahead of print. J Nephrol. 2025. PMID: 40349276 Review.
References
-
- Chesnaye N, Bonthuis M, Schaefer Fet al. . Demographics of paediatric renal replacement therapy in Europe: a report of the ESPN/ERA-EDTA registry. Pediatr Nephrol 2014; 29: 2403–2410 - PubMed
-
- Atkinson MA, Martz K, Warady BAet al. . Risk for anemia in pediatric chronic kidney disease patients: a report of NAPRTCS. Pediatr Nephrol 2010; 25: 1699–1706 - PubMed
-
- Wong H, Mylrea K, Feber Jet al. . Prevalence of complications in children with chronic kidney disease according to KDOQI. Kidney Int 2006; 70: 585–590 - PubMed
Publication types
LinkOut - more resources
Full Text Sources