DLEU7-AS1 promotes renal cell cancer by silencing the miR-26a-5p/coronin-3 axis
- PMID: 35892027
- PMCID: PMC9308101
- DOI: 10.1093/ckj/sfac061
DLEU7-AS1 promotes renal cell cancer by silencing the miR-26a-5p/coronin-3 axis
Abstract
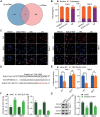
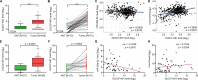
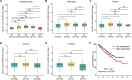
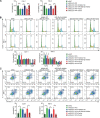
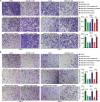
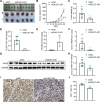
Long non-coding RNAs (lncRNAs) have been implicated in the progression and development of many types of cancer by interacting with RNA, DNA and proteins, including DLEU7-AS1. However, the function of DLEU7-AS1 in renal cell cancer (RCC) remains unclear. In this study, two in silico prediction algorithms were used to discover the potential target of miR-26a-5p, which was determined to be a tumor suppressor gene, possibly DLEU7-AS1, through the downregulation of coronin-3 in RCC. Thus, we hypothesized that DLEU7-AS1 promotes RCC by silencing the miR-26a-5p/coronin-3 axis. To test our hypothesis, we confirmed that DLEU7-AS1 directly targets miR-26a-5p using the pmirGLO dual-luciferase reporter assay. Next, we observed that DLEU7-AS1 expression was markedly upregulated in RCC samples and inversely correlated with clinical prognosis and miR-26a-5p levels. Knockdown of DLEU7-AS1 significantly suppressed the growth and metastasis of RCC cells in vitro and attenuated tumor growth in vivo. Interestingly, exogenous expression of coronin-3 or miR-26a-5p inhibitor treatment almost completely rescued the DLEU7-AS1 knockdown-induced inhibitory effects on cell proliferation, migration and invasion. In conclusion, our data demonstrate that DLEU7-AS1 is an oncogene in RCC capable of regulating the growth and metastasis of RCC by silencing the miR-26a-5p/coronin-3 axis, suggesting that DLEU7-AS1 can be employed as a potential therapeutic target and prognostic biomarker for RCC.
Keywords: DLEU7-AS1; coronin-3; miR-26a-5p; renal cell cancer.
© The Author(s) 2022. Published by Oxford University Press on behalf of the ERA.
Figures

References
-
- Sung H, Ferlay Jet al. . Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians2021; 71(3): 209–249 - PubMed
-
- Barata PC, Rini BI. Treatment of renal cell carcinoma: current status and future directions. CA Cancer J Clin 2017; 67: 507–524 - PubMed
LinkOut - more resources
Full Text Sources

