Vasorin plays a critical role in vascular smooth muscle cells and arterial functions
- PMID: 35892191
- PMCID: PMC9796581
- DOI: 10.1002/jcp.30838
Vasorin plays a critical role in vascular smooth muscle cells and arterial functions
Abstract
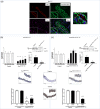
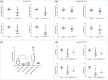
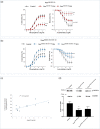
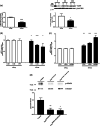
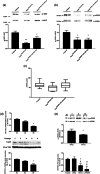
Within the cardiovascular system, the protein vasorin (Vasn) is predominantly expressed by vascular smooth muscle cells (VSMCs) in the coronary arteries and the aorta. Vasn knockout (Vasn-/- ) mice die within 3 weeks of birth. In the present study, we investigated the role of vascular Vasn expression on vascular function. We used inducible Vasn knockout mice (VasnCRE-ERT KO and VasnSMMHC-CRE-ERT2 KO , in which respectively all cells or SMCs only are targeted) to analyze the consequences of total or selective Vasn loss on vascular function. Furthermore, in vivo effects were investigated in vitro using human VSMCs. The death of VasnCRE-ERT KO mice 21 days after tamoxifen injection was concomitant with decreases in blood pressure, angiotensin II levels, and vessel contractibility to phenylephrine. The VasnSMMHC-CRE-ERT2 KO mice displayed concomitant changes in vessel contractibility in response to phenylephrine and angiotensin II levels. In vitro, VASN deficiency was associated with a shift toward the SMC contractile phenotype, an increase in basal intracellular Ca2+ levels, and a decrease in the SMCs' ability to generate a calcium signal in response to carbachol or phenylephrine. Additionally, impaired endothelium-dependent relaxation (due to changes in nitric oxide signaling) was observed in all Vasn knockout mice models. Our present findings highlight the role played by Vasn SMC expression in the maintenance of vascular functions. The mechanistic experiments suggested that these effects are mediated by SMC phenotype switching and changes in intracellular calcium homeostasis, angiotensin II levels, and NO signaling.
Keywords: angiotensin II; artery; nitric oxide; smooth muscle cells; vascular function; vasorin.
© 2022 The Authors. Journal of Cellular Physiology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Bravo‐Sagua, R. , Parra, V. , Muñoz‐Cordova, F. , Sanchez‐Aguilera, P. , Garrido, V. , Contreras‐Ferrat, A. , Chiong, M. , & Lavandero, S. (2020). Sarcoplasmic reticulum and calcium signaling in muscle cells: Homeostasis and disease. International Review of Cell and Molecular Biology, 350, 197–264. 10.1016/bs.ircmb.2019.12.007 - DOI - PubMed
-
- Choksi, S. , Lin, Y. , Pobezinskaya, Y. , Chen, L. , Park, C. , Morgan, M. , Li, T. , Jitkaew, S. , Cao, X. , Kim, Y. S. , Kim, H. S. , Levitt, P. , Shih, G. , Birre, M. , Deng, C. X. , & Liu, Z. G. (2011). A HIF‐1 target, ATIA, protects cells from apoptosis by modulating the mitochondrial thioredoxin, TRX2. Molecular Cell, 42(5), 597–609. 10.1016/j.molcel.2011.03.030 - DOI - PMC - PubMed
-
- Daya, H. A. , Kouba, S. , Ouled‐Haddou, H. , Benzerdjeb, N. , Telliez, M. S. , Dayen, C. , Sevestre, H. , Garçon, L. , Hague, F. , & Ouadid‐Ahidouch, H. (2021). Orai3‐mediates cisplatin‐resistance in non‐small cell lung cancer cells by enriching cancer stem cell population through PI3K/AKT pathway. Cancers, 13(10), 2314. 10.3390/cancers13102314 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

