Endocrine cybernetics: neuropeptides as molecular switches in behavioural decisions
- PMID: 35892199
- PMCID: PMC9326288
- DOI: 10.1098/rsob.220174
Endocrine cybernetics: neuropeptides as molecular switches in behavioural decisions
Abstract
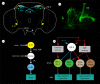
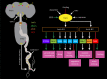
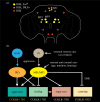
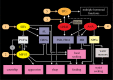
Plasticity in animal behaviour relies on the ability to integrate external and internal cues from the changing environment and hence modulate activity in synaptic circuits of the brain. This context-dependent neuromodulation is largely based on non-synaptic signalling with neuropeptides. Here, we describe select peptidergic systems in the Drosophila brain that act at different levels of a hierarchy to modulate behaviour and associated physiology. These systems modulate circuits in brain regions, such as the central complex and the mushroom bodies, which supervise specific behaviours. At the top level of the hierarchy there are small numbers of large peptidergic neurons that arborize widely in multiple areas of the brain to orchestrate or modulate global activity in a state and context-dependent manner. At the bottom level local peptidergic neurons provide executive neuromodulation of sensory gain and intrinsically in restricted parts of specific neuronal circuits. The orchestrating neurons receive interoceptive signals that mediate energy and sleep homeostasis, metabolic state and circadian timing, as well as external cues that affect food search, aggression or mating. Some of these cues can be triggers of conflicting behaviours such as mating versus aggression, or sleep versus feeding, and peptidergic neurons participate in circuits, enabling behaviour choices and switches.
Keywords: Drosophila melanogaster; brain circuits; interneurons; neuromodulation; peptide hormones.
Conflict of interest statement
We declare we have no competing interests.
Figures
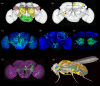
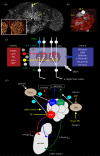
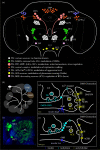
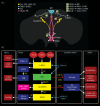


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
