Development and characterization of anti-SARS-CoV-2 intravenous immunoglobulin from COVID-19 convalescent plasma
- PMID: 35892311
- PMCID: PMC9328115
- DOI: 10.2217/imt-2022-0015
Development and characterization of anti-SARS-CoV-2 intravenous immunoglobulin from COVID-19 convalescent plasma
Abstract
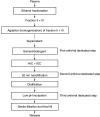
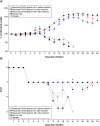
Background: The authors describe the developmental process of intravenous anti-COVID-19 hyperimmune immunoglobulin from anti-SARS-CoV-2 neutralizing antibody-containing plasma. Furthermore, the authors investigated its safety and protective activity in animal models. Materials & methods: The manufacturing process included standard ethanol fractionation, chromatographic purification steps and virus removal or inactivation. Results: The authors produced pure and safe immunoglobulin for intravenous administration, with 98.1 ± 6.5 mg/ml protein content, of which 97.6 ± 0.7% was IgG. The concentration factor of SARS-CoV-2 neutralizing antibodies was 9.4 ± 1.4-times. Safety studies in animals showed no signs of acute/chronic toxicity or allergenic or thrombogenic properties. Intravenous anti-COVID-19 hyperimmune immunoglobulin protected immunosuppressed hamsters against SARS-Cov-2. Conclusion: The obtained results can allow the start of clinical trials to study the safety and efficacy in healthy adults.
Keywords: COVID-globulin; IgG; SARS-CoV-2; hyperimmune immunoglobulin; immunoglobulin against COVID-19; preclinical evaluation; protective efficacy.
Plain language summary
An intravenous immunoglobulin with a high concentration of SARS-CoV-2-neutralizing antibodies was prepared from COVID-19 convalescent plasma, which could be utilized as a passive immunization tool in regard to COVID-19 treatment. The manufacturing process employed conforms to commonly held business standards within the intravenous immunoglobulin industry and includes plasma ethanol fractionation following chromatographic purification and special virus removal or inactivation steps. The results of the preclinical in vitro and in vivo experiments demonstrate that the immunoglobulin produced in this study is pure and safe enough to be considered for intravenous applications. The SARS-CoV-2 neutralizing antibody concentration was found to have increased 9.4 ± 1.4-times compared with human plasma. The anti-COVID-19 hyperimmune immunoglobulin showed no signs of toxicity and did not cause any blood clot formations when administered to rabbits. Furthermore, the anti-COVID-19 hyperimmune immunoglobulin was demonstrated to protect immunosuppressed hamsters against SARS-CoV-2.
Conflict of interest statement
This work was sponsored and performed by JSC Nacimbio. The employees of Nacimbio, M Razumikhin and T Smolyanova, participated in and contributed to the study. However, the company Nacimbio as a funding source had no direct role in the study design; in the collection, analysis or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
This manuscript was prepared with the help of Editage Publication Support. Writing assistance was sponsored by JSC Nacimbio.
Figures


References
-
- Worldometer. COVID-19 coronavirus pandemic. www.worldometers.info/coronavirus/
-
- Mupapa K, Massamba M, Kibadi K et al. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. International Scientific and Technical Committee. J. Infect. Dis. 179(1), 18–23 (1999). - PubMed
-
- Focosi D, Franchini M, Pirofski LA et al. COVID-19 convalescent plasma is more than neutralizing antibodies: a narrative review of potential beneficial and detrimental co-factors. Viruses 13(8), 1594 (2021). - PMC - PubMed
-
• Provided the analysis of potential beneficial and detrimental factors of COVID-19 convalescent plasma.
-
- Yuwono Soeroto A, Purwiga A, Alam A, Prasetya D. Plasma convalescent decrease mortality in COVID-19 patients: a systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 25(14), 4841–4853 (2021). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
