Population Pharmacokinetic and Pharmacodynamic Analysis of Dalbavancin for Long-Term Treatment of Subacute and/or Chronic Infectious Diseases: The Major Role of Therapeutic Drug Monitoring
- PMID: 35892386
- PMCID: PMC9331863
- DOI: 10.3390/antibiotics11080996
Population Pharmacokinetic and Pharmacodynamic Analysis of Dalbavancin for Long-Term Treatment of Subacute and/or Chronic Infectious Diseases: The Major Role of Therapeutic Drug Monitoring
Abstract
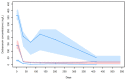
A population pharmacokinetic analysis of dalbavancin was conducted in patients with different infection sites. Non-linear mixed effect modeling was used for pharmacokinetic analysis and covariate evaluation. Monte Carlo simulations assessed the probability of target attainment (PTA) of total dalbavancin concentration ≥ 8.04 mg/L over time (associated with ≥90% probability of optimal pharmacodynamic target attainment of fAUC24h/MIC > 111.1 against S. aureus) associated with a single or double dosage, one week apart, of 1000 or 1500 mg in patients with different classes of renal function. Sixty-nine patients with 289 concentrations were included. Most of them (53/69, 76.8%) had bone and joint infections. A two-compartment model adequately fitted dalbavancin concentration−time data. Creatinine clearance (CLCR) was the only covariate associated with dalbavancin clearance. Monte Carlo simulations showed that, in patients with severe renal dysfunction, the 1000 mg single or double one week apart dosage may ensure optimal PTAs of 2 and 5 weeks, respectively. In patients with preserved renal function, the 1500 mg single or double one-week apart dosage may ensure optimal PTAs of 2 and 4 to 6 weeks, respectively. Therapeutic drug monitoring should be considered mandatory for managing inter-individual variability and for supporting clinicians in long-term treatments of subacute and chronic infections.
Keywords: dalbavancin; long-term treatment; off-label use; population pharmacokinetics; therapeutic drug monitoring.
Conflict of interest statement
P.G.C. has received personal fees from Angelini and Shianogi. M.G. has received personal fees from Angelini and Shianogi. CT has received personal fees from Astra, Merck, Pfizer, Angelini, bioMérieux, Thermofischer, Zabon, Hikma, Avir-Pharma, Shionogi, Biotest. AMC received travel grants by Angelini. S.T. has received personal fees from Shianogi. FP has participated in speaker bureaus for Angelini, Basilea Pharmaceutica, Gilead, Hikma, Merck Sharp and Dohme, Nordic Pharma, Pfizer and Sanofi Aventis, and in advisory boards for Angelini, Basilea, Pharmaceutica, Correvio, Gilead, Hikma, Merck Sharp and Dohme, Nordic Pharma, Novartis, Pfizer, Shionogi and Thermo-Fisher, outside the submitted work. PV has served as a consultant for bioMérieux, Gilead, Merck Sharp and Dohme, Nabriva, Nordic Pharma, Pfizer, Thermo-Fisher, and Venatorx, and received payment for serving on the speaker’s bureaus for Correvio, Gilead, Merck Sharp and Dohme, Nordic Pharma, and Pfizer, outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources

