A Criterion of Colorectal Cancer Diagnosis Using Exosome Fluorescence-Lifetime Imaging
- PMID: 35892503
- PMCID: PMC9394250
- DOI: 10.3390/diagnostics12081792
A Criterion of Colorectal Cancer Diagnosis Using Exosome Fluorescence-Lifetime Imaging
Abstract
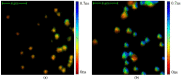
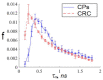
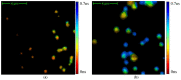
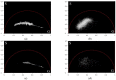
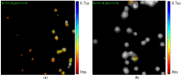
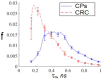
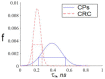
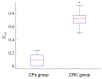
This study was aimed to investigate the applicability of the exosome fluorescence-lifetime imaging microscopy (FLIM) for colorectal cancer (CRC) diagnosis. Differential ultra-centrifugation was used to extract exosomes from the blood plasma of 11 patients with colon polyps (CPs) and 13 patients with CRC at the T2-4, N0-3, and M0-1 stages. Analysis was performed using a two-photon FLIM device. In total, 165 and 195 FLIM images were recorded for the CP and CCR patient groups, respectively. Two classes of exosomes differentiated by autofluorescence average lifetime tm were discovered in the samples. The first class of exosomes with tm = (0.21 ± 0.06) ns was mostly found in samples from CRC patients. The second class with tm = (0.43 ± 0.19) ns was mostly found in samples from CP patients. The relative number of “CRC-associated” exosomes Nch in the FLIM dataset was shown to be very small for the CP patient group and large for the CRC patient group. This difference was statistically significant. Therefore, the suggested CRS diagnostics criterion can be as follows. If Nch > 0.5, the probability of CRC is high. If Nch < 0.3, the probability of CRC is low.
Keywords: colorectal cancer; exosomes; fluorescence-lifetime imaging microscopy; two-photon exited autofluorescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hossain M.S., Karuniawati H., Jairoun A.A., Urbi Z., Ooi J., John A., Lim Y.C., Kibria K.M.K., Mohiuddin A.K.M., Ming L.C., et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers. 2022;14:1732. doi: 10.3390/cancers14071732. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

