A 3D In Vivo Model for Studying Human Renal Cystic Tissue and Mouse Kidney Slices
- PMID: 35892566
- PMCID: PMC9330914
- DOI: 10.3390/cells11152269
A 3D In Vivo Model for Studying Human Renal Cystic Tissue and Mouse Kidney Slices
Abstract
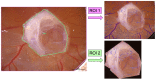
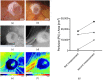
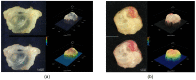
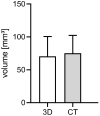
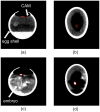
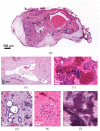
(1) Background: Autosomal dominant polycystic kidney disease (ADPKD) is a frequent monogenic disorder that leads to progressive renal cyst growth and renal failure. Strategies to inhibit cyst growth in non-human cyst models have often failed in clinical trials. There is a significant need for models that enable studies of human cyst growth and drug trials. (2) Methods: Renal tissue from ADPKD patients who received a nephrectomy as well as adult mouse kidney slices were cultured on a chorioallantoic membrane (CAM) for one week. The cyst volume was monitored by microscopic and CT-based applications. The weight and angiogenesis were quantified. Morphometric and histological analyses were performed after the removal of the tissues from the CAM. (3) Results: The mouse and human renal tissue mostly remained vital for about one week on the CAM. The growth of cystic tissue was evaluated using microscopic and CT-based volume measurements, which correlated with weight and an increase in angiogenesis, and was accompanied by cyst cell proliferation. (4) Conclusions: The CAM model might bridge the gap between animal studies and clinical trials of human cyst growth, and provide a drug-testing platform for the inhibition of cyst enlargement. Real-time analyses of mouse kidney tissue may provide insights into renal physiology and reduce the need for animal experiments.
Keywords: 3D in vivo model; ADPKD; chorioallantoic membrane (CAM) model; human renal cystic tissue; mouse kidney slices; polycystic kidney disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

