Pseudomonas aeruginosa Alters Critical Lung Epithelial Cell Functions through Activation of ADAM17
- PMID: 35892600
- PMCID: PMC9331763
- DOI: 10.3390/cells11152303
Pseudomonas aeruginosa Alters Critical Lung Epithelial Cell Functions through Activation of ADAM17
Abstract
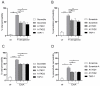
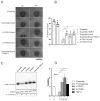
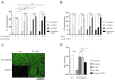
Severe epithelial dysfunction is one major hallmark throughout the pathophysiological progress of bacterial pneumonia. Junctional and cellular adhesion molecules (e.g., JAMA-A, ICAM-1), cytokines (e.g., TNFα), and growth factors (e.g., TGFα), controlling proper lung barrier function and leukocyte recruitment, are proteolytically cleaved and released into the extracellular space through a disintegrin and metalloproteinase (ADAM) 17. In cell-based assays, we could show that the protein expression, maturation, and activation of ADAM17 is upregulated upon infection of lung epithelial cells with Pseudomonas aeruginosa and Exotoxin A (ExoA), without any impact of infection by Streptococcus pneumoniae. The characterization of released extracellular vesicles/exosomes and the comparison to heat-inactivated bacteria revealed that this increase occurred in a cell-associated and toxin-dependent manner. Pharmacological targeting and gene silencing of ADAM17 showed that its activation during infection with Pseudomonas aeruginosa was critical for the cleavage of junctional adhesion molecule A (JAM-A) and epithelial cell survival, both modulating barrier integrity, epithelial regeneration, leukocyte adhesion and transepithelial migration. Thus, site-specific targeting of ADAM17 or blockage of the activating toxins may constitute a novel anti-infective therapeutic option in Pseudomonas aeruginosa lung infection preventing severe epithelial and organ dysfunctions and stimulating future translational studies.
Keywords: exosomes; junctional molecules; lung infection; metalloproteinase; proteolysis; regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Bredikis I.I., Bukauskas F.F., Liakaia R.I., Zhebrauskas R.I., Laurushonis K.A. Method of epicardial electrocartography of the heart during surgical treatment of Wolff-Parkinson-White syndrome. Grud. Khirurgiia Mosc. Russ. 1982;6:31–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

