Development of In Vitro Assays for Advancing Radioimmunotherapy against Brain Tumors
- PMID: 35892697
- PMCID: PMC9394411
- DOI: 10.3390/biomedicines10081796
Development of In Vitro Assays for Advancing Radioimmunotherapy against Brain Tumors
Abstract
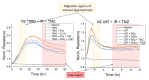
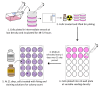
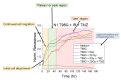
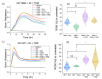
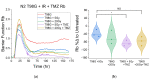
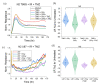
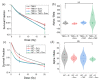
Glioblastoma (GBM) is the most common primary brain tumor. Due to high resistance to treatment, local invasion, and a high risk of recurrence, GBM patient prognoses are often dismal, with median survival around 15 months. The current standard of care is threefold: surgery, radiation therapy, and chemotherapy with temozolomide (TMZ). However, patient survival has only marginally improved. Radioimmunotherapy (RIT) is a fourth modality under clinical trials and aims at combining immunotherapeutic agents with radiotherapy. Here, we develop in vitro assays for the rapid evaluation of RIT strategies. Using a standard cell irradiator and an Electric Cell Impedance Sensor, we quantify cell migration following the combination of radiotherapy and chemotherapy with TMZ and RIT with durvalumab, a PD-L1 immune checkpoint inhibitor. We measure cell survival using a cloud-based clonogenic assay. Irradiated T98G and U87 GBM cells migrate significantly (p < 0.05) more than untreated cells in the first 20−40 h post-treatment. Addition of TMZ increases migration rates for T98G at 20 Gy (p < 0.01). Neither TMZ nor durvalumab significantly change cell survival in 21 days post-treatment. Interestingly, durvalumab abolishes the enhanced migration effect, indicating possible potency against local invasion. These results provide parameters for the rapid supplementary evaluation of RIT against brain tumors.
Keywords: brain cancers; durvalumab; glioblastoma; immune checkpoint inhibitors; immunoradiotherapy; immunotherapy; radioimmunotherapy; radiotherapy; temozolomide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

