Adverse Pathological Findings at Radical Prostatectomy following Active Surveillance: Results from the Movember GAP3 Cohort
- PMID: 35892817
- PMCID: PMC9332009
- DOI: 10.3390/cancers14153558
Adverse Pathological Findings at Radical Prostatectomy following Active Surveillance: Results from the Movember GAP3 Cohort
Abstract
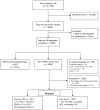
Background: Little is known about the consequences of delaying radical prostatectomy (RP) after Active Surveillance (AS) according to stringent or wider entry criteria. We investigated the association between inclusion criteria and rates, and timing of adverse pathological findings (APFs) among patients in GAP3 cohorts. Methods: APFs (GG ≥ 3, pT ≥ 3, pN > 0 and positive surgical margins [R1]) were accounted for in very low-risk (VLR: grade group [GG] 1, cT1, positive cores < 3, PSA < 10 ng/mL, PSA density [PSAD] < 0.15 ng/mL/cm3) and low-risk (LR: GG1, cT1-2, PSA ≤ 10 ng/mL) patients undergoing subsequent RP. The Kaplan−Meier method and log−rank test analyzed APF-free survival. Stratified mixed effects models analyzed association. Results: Out of 21,169 patients on AS, 1742 (VLR: 721; LR: 1021) underwent delayed RP. Most (60.8%) did not have APFs. APFs occurred more frequently (44.6% vs. 31.7%; OR 1.54, p < 0.001) and earlier (median time: 40.3 vs. 62.6 months; p < 0.001) in LR patients, and consisted of pT ≥ 3 (OR 1.47, p = 0.013) or R1 (OR 1.80, p < 0.001), but not of GG ≥ 3 or node involvement. Age (OR 1.05, p < 0.001), PSAD (OR 23.21, p = 0.003), and number of positive cores (OR 1.16, p = 0.004) were independently associated with APFs. Conclusions: AS stands as a safe option for low-risk patients, and most do not have APFs at surgery. Wider entry criteria are associated with pT3 and R1. The prognostic implications remain uncertain.
Keywords: active surveillance; classification; outcome; pathology; prognosis; prostatectomy; prostatic neoplasms; risk assessment; watchful waiting.
Conflict of interest statement
Paul C. Boutros sits on the scientific advisory boards of Sage Bionetworks, Intersect Diagnostics Inc., and BioSymetrics Inc. The other authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Lardas M., Liew M., Van den Bergh R.C., De Santis M., Bellmunt J., Van den Broeck T., Cornford P., Cumberbatch M.G., Fossati N., Gross T., et al. Quality of Life Outcomes after Primary Treatment for Clinically Localised Prostate Cancer: A Systematic Review. Eur. Urol. 2017;72:869–885. doi: 10.1016/j.eururo.2017.06.035. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous