Proton Beam Therapy versus Photon Radiotherapy for Stage I Non-Small Cell Lung Cancer
- PMID: 35892885
- PMCID: PMC9329768
- DOI: 10.3390/cancers14153627
Proton Beam Therapy versus Photon Radiotherapy for Stage I Non-Small Cell Lung Cancer
Abstract
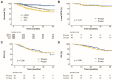
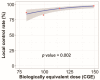
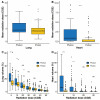
Proton beam therapy (PBT) and photon radiotherapy for stage I non-small cell lung cancer (NSCLC) were compared in terms of clinical outcomes and dosimetry. Data were obtained from patients who underwent PBT or photon radiotherapy at two institutions-the only two facilities where PBT is available in the Republic of Korea. Multivariate Cox proportional hazards models and propensity score-matched analyses were used to compare local progression-free survival (PFS) and overall survival (OS). Survival and radiation exposure to the lungs were compared in the matched population. Of 289 patients included in the analyses, 112 and 177 underwent PBT and photon radiotherapy, respectively. With a median follow-up duration of 27 months, the 2-year local PFS and OS rates were 94.0% and 83.0%, respectively. In the multivariate analysis, a biologically effective dose (BED10, using α/β = 10 Gy) of ≥125 cobalt gray equivalents was significantly associated with improved local PFS and OS. In the matched analyses, the local PFS and OS did not differ between groups. However, PBT showed significantly lower lung and heart radiation exposure in the mean dose, V5, and V10 than photon radiotherapy. PBT significantly reduced radiation exposure to the heart and lungs without worsening disease control in stage I NSCLC patients.
Keywords: dosimetric comparison; hypofractionated radiotherapy; non-small cell lung cancer; proton beam therapy; stereotactic ablative radiotherapy; stereotactic body radiotherapy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Baumann P., Nyman J., Hoyer M., Wennberg B., Gagliardi G., Lax I., Drugge N., Ekberg L., Friesland S., Johansson K.-A., et al. Outcome in a Prospective Phase II Trial of Medically Inoperable Stage I Non–Small-Cell Lung Cancer Patients Treated With Stereotactic Body Radiotherapy. J. Clin. Oncol. 2009;27:3290–3296. doi: 10.1200/JCO.2008.21.5681. - DOI - PubMed
-
- Videtic G.M., Hu C., Singh A.K., Chang J.Y., Parker W., Olivier K.R., Schild S.E., Komaki R., Urbanic J.J., Timmerman R.D., et al. A Randomized Phase 2 Study Comparing 2 Stereotactic Body Radiation Therapy Schedules for Medically Inoperable Patients With Stage I Peripheral Non-Small Cell Lung Cancer: NRG Oncology RTOG 0915 (NCCTG N0927) Int. J. Radiat. Oncol. Biol. Phys. 2015;93:757–764. doi: 10.1016/j.ijrobp.2015.07.2260. - DOI - PMC - PubMed
-
- Timmerman R., McGarry R., Yiannoutsos C., Papiez L., Tudor K., DeLuca J., Ewing M., Abdulrahman R., DesRosiers C., Williams M., et al. Excessive Toxicity When Treating Central Tumors in a phase II Study of Stereotactic Body Radiation Therapy for Medically Inoperable Early-Stage Lung Cancer. J. Clin. Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. - DOI - PubMed
-
- Lindberg K., Grozman V., Karlsson K., Lindberg S., Lax I., Wersäll P., Persson G.F., Josipovic M., Khalil A.A., Moeller D.S., et al. The HILUS-Trial-A Prospective Nordic Multicenter Phase 2 Study of Ultracentral Lung Tumors Treated With Ste-reotactic Body Radiotherapy. J. Thorac. Oncol. 2021;16:1200–1210. doi: 10.1016/j.jtho.2021.03.019. - DOI - PubMed
Grants and funding
- NCC 2110590/National Cancer Center/Republic of Korea
- HC19C0293/the Korea Health Technology R&D Project of the Korea Health Industry development Institute (KHIDI) funded by Ministry of Health and Wellfare, Republic of Korea
- HI19C0481/the Korea Health Technology R&D Project of the Korea Health Industry development Institute (KHIDI) funded by Ministry of Health and Wellfare, Republic of Korea
LinkOut - more resources
Full Text Sources

