Anthraquinones and Their Analogues from Marine-Derived Fungi: Chemistry and Biological Activities
- PMID: 35892942
- PMCID: PMC9394430
- DOI: 10.3390/md20080474
Anthraquinones and Their Analogues from Marine-Derived Fungi: Chemistry and Biological Activities
Abstract
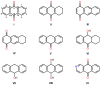
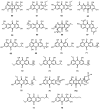
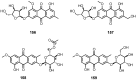
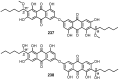
Anthraquinones are an interesting chemical class of polyketides since they not only exhibit a myriad of biological activities but also contribute to managing ecological roles. In this review article, we provide a current knowledge on the anthraquinoids reported from marine-derived fungi, isolated from various resources in both shallow waters such as mangrove plants and sediments of the mangrove habitat, coral reef, algae, sponges, and deep sea. This review also tentatively categorizes anthraquinone metabolites from the simplest to the most complicated scaffolds such as conjugated xanthone-anthraquinone derivatives and bianthraquinones, which have been isolated from marine-derived fungi, especially from the genera Apergillus, Penicillium, Eurotium, Altenaria, Fusarium, Stemphylium, Trichoderma, Acremonium, and other fungal strains. The present review, covering a range from 2000 to 2021, was elaborated through a comprehensive literature search using the following databases: ACS publications, Elsevier, Taylor and Francis, Wiley Online Library, MDPI, Springer, and Thieme. Thereupon, we have summarized and categorized 296 anthraquinones and their derivatives, some of which showed a variety of biological properties such as enzyme inhibition, antibacterial, antifungal, antiviral, antitubercular (against Mycobacterium tuberculosis), cytotoxic, anti-inflammatory, antifouling, and antioxidant activities. In addition, proposed biogenetic pathways of some anthraquinone derivatives are also discussed.
Keywords: Aspergillus sp.; Penicillium sp.; anthraquinones; antibacterial activity; bianthraquinones; cytotoxicity; hydroanthraquinones; marine-derived fungi.
Conflict of interest statement
The authors declare no conflict of interest.
Figures























References
-
- Kis-papo T. Marine fungal communities. In: Dighton J., White J.F., Oudemans P., editors. The Fungal Community: Its Organization and Role in the Ecosystem. 3rd ed. Volume 23. CRC Press; Boca Raton, FL, USA: Taylor & Francis Group; Oxfordshire, UK: 2005. pp. 61–92.
-
- Kohlmeyer J., Kohlmeyer E. Marine Mycology, the Higher Fungi. Academic Press; New York, NY, USA: 1979. pp. 1–3.
-
- Pang K.-L., Overy D.P., Jones E.G., da Luz Calado M., Burgaud G., Walker A.K., Johnson J.A., Kerr R.G., Cha H.-J., Bills G.F. ‘Marine fungi’and ‘marine-derived fungi’in natural product chemistry research: Toward a new consensual definition. Fungal Biol. Rev. 2016;30:163–175. doi: 10.1016/j.fbr.2016.08.001. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

