Adipokines in Sleep Disturbance and Metabolic Dysfunction: Insights from Network Analysis
- PMID: 35892989
- PMCID: PMC9326621
- DOI: 10.3390/clockssleep4030027
Adipokines in Sleep Disturbance and Metabolic Dysfunction: Insights from Network Analysis
Abstract
Adipokines are a growing group of secreted proteins that play important roles in obesity, sleep disturbance, and metabolic derangements. Due to the complex interplay between adipokines, sleep, and metabolic regulation, an integrated approach is required to better understand the significance of adipokines in these processes. In the present study, we created and analyzed a network of six adipokines and their molecular partners involved in sleep disturbance and metabolic dysregulation. This network represents information flow from regulatory factors, adipokines, and physiologic pathways to disease processes in metabolic dysfunction. Analyses using network metrics revealed that obesity and obstructive sleep apnea were major drivers for the sleep associated metabolic dysregulation. Two adipokines, leptin and adiponectin, were found to have higher degrees than other adipokines, indicating their central roles in the network. These adipokines signal through major metabolic pathways such as insulin signaling, inflammation, food intake, and energy expenditure, and exert their functions in cardiovascular, reproductive, and autoimmune diseases. Leptin, AMP activated protein kinase (AMPK), and fatty acid oxidation were found to have global influence in the network and represent potentially important interventional targets for metabolic and sleep disorders. These findings underscore the great potential of using network based approaches to identify new insights and pharmaceutical targets in metabolic and sleep disorders.
Keywords: adipokine; cardiovascular disease; metabolic syndrome; network analysis; sleep disorder.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
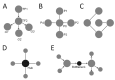
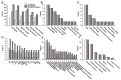

References
-
- Jullian-Desayes I., Joyeux-Faure M., Tamisier R., Launois S., Borel A.L., Levy P., Pepin J.L. Impact of obstructive sleep apnea treatment by continuous positive airway pressure on cardiometabolic biomarkers: A systematic review from sham CPAP randomized controlled trials. Sleep Med. Rev. 2015;21:23–38. doi: 10.1016/j.smrv.2014.07.004. - DOI - PubMed
-
- Vgontzas A.N., Papanicolaou D.A., Bixler E.O., Hopper K., Lotsikas A., Lin H.M., Kales A., Chrousos G.P. Sleep apnea and daytime sleepiness and fatigue: Relation to visceral obesity, insulin resistance, and hypercytokinemia. J. Clin. Endocrinol. Metab. 2000;85:1151–1158. doi: 10.1210/jcem.85.3.6484. - DOI - PubMed
LinkOut - more resources
Full Text Sources

