BAG3 Alleviates Atherosclerosis by Inhibiting Endothelial-to-Mesenchymal Transition via Autophagy Activation
- PMID: 35893075
- PMCID: PMC9332509
- DOI: 10.3390/genes13081338
BAG3 Alleviates Atherosclerosis by Inhibiting Endothelial-to-Mesenchymal Transition via Autophagy Activation
Abstract
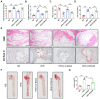
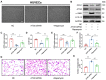
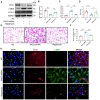
Atherosclerosis is a chronic systemic inflammatory disease that causes severe cardiovascular events. B cell lymphoma 2-associated athanogene (BAG3) was proven to participate in the regulation of tumor angiogenesis, neurodegenerative diseases, and cardiac diseases, but its role in atherosclerosis remains unclear. Here, we aim to investigate the role of BAG3 in atherosclerosis and elucidate the potential molecular mechanism. In this study, ApoE-/- mice were given a tail-vein injection of BAG3-overexpressing lentivirus and fed a 12-week high-fat diet (HFD) to investigate the role of BAG3 in atherosclerosis. The overexpression of BAG3 reduced plaque areas and improved atherosclerosis in ApoE-/- mice. Our research proves that BAG3 promotes autophagy in vitro, contributing to the suppression of EndMT in human umbilical vein endothelial cells (HUVECs). Mechanically, autophagy activation is mediated by BAG3 via the interaction between BAG3 and its chaperones HSP70 and HSPB8. In conclusion, BAG3 facilitates autophagy activation via the formation of the chaperone-assisted selective autophagy (CASA) complex interacting with HSP70 and HSPB8, leading to the inhibition of EndMT during the progression of atherosclerosis and indicating that BAG3 is a potential therapeutic target for atherosclerosis.
Keywords: BAG3; CASA complex; EndMT; atherosclerosis; autophagy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
HSPB8 frameshift mutant aggregates weaken chaperone-assisted selective autophagy in neuromyopathies.Autophagy. 2023 Aug;19(8):2217-2239. doi: 10.1080/15548627.2023.2179780. Epub 2023 Feb 28. Autophagy. 2023. PMID: 36854646 Free PMC article.
-
HSPB8-BAG3 chaperone complex modulates cell invasion in intrahepatic cholangiocarcinoma by regulating CASA-mediated Filamin A degradation.Cancer Biol Ther. 2024 Dec 31;25(1):2396694. doi: 10.1080/15384047.2024.2396694. Epub 2024 Aug 31. Cancer Biol Ther. 2024. PMID: 39215616 Free PMC article.
-
BAG3 Pro209 mutants associated with myopathy and neuropathy relocate chaperones of the CASA-complex to aggresomes.Sci Rep. 2020 May 29;10(1):8755. doi: 10.1038/s41598-020-65664-z. Sci Rep. 2020. PMID: 32472079 Free PMC article.
-
Universal Adapter Protein Bag3 and Small Heat Shock Proteins.Biochemistry (Mosc). 2024 Sep;89(9):1535-1545. doi: 10.1134/S0006297924090013. Biochemistry (Mosc). 2024. PMID: 39418513 Review.
-
The role of BAG3 in dilated cardiomyopathy and its association with Charcot-Marie-Tooth disease type 2.Acta Myol. 2022 Jun 30;41(2):59-75. doi: 10.36185/2532-1900-071. eCollection 2022 Jun. Acta Myol. 2022. PMID: 35832504 Free PMC article. Review.
Cited by
-
Editorial for the Molecular Genetics and Genomics of Metabolic Disorders in Cardiovascular and Cerebrovascular Diseases Special Issue: June 2023.Genes (Basel). 2023 Aug 1;14(8):1568. doi: 10.3390/genes14081568. Genes (Basel). 2023. PMID: 37628620 Free PMC article.
-
Elucidating the crosstalk between endothelial-to-mesenchymal transition (EndoMT) and endothelial autophagy in the pathogenesis of atherosclerosis.Vascul Pharmacol. 2024 Jun;155:107368. doi: 10.1016/j.vph.2024.107368. Epub 2024 Mar 26. Vascul Pharmacol. 2024. PMID: 38548093 Free PMC article. Review.
-
The Role of Alarmins in the Pathogenesis of Atherosclerosis and Myocardial Infarction.Curr Issues Mol Biol. 2024 Aug 17;46(8):8995-9015. doi: 10.3390/cimb46080532. Curr Issues Mol Biol. 2024. PMID: 39194749 Free PMC article. Review.
-
Functional Diversity of Mammalian Small Heat Shock Proteins: A Review.Cells. 2023 Jul 27;12(15):1947. doi: 10.3390/cells12151947. Cells. 2023. PMID: 37566026 Free PMC article. Review.
-
MiR-483-5p downregulation alleviates ox-LDL induced endothelial cell injury in atherosclerosis.BMC Cardiovasc Disord. 2023 Oct 27;23(1):521. doi: 10.1186/s12872-023-03496-1. BMC Cardiovasc Disord. 2023. PMID: 37891465 Free PMC article.
References
-
- Giordo R., Ahmed Y.M.A., Allam H., Abusnana S., Pappalardo L., Nasrallah G.K., Mangoni A.A., Pintus G. EndMT Regulation by Small RNAs in Diabetes-Associated Fibrotic Conditions: Potential Link with Oxidative Stress. Front. Cell Dev. Biol. 2021;9:683594. doi: 10.3389/fcell.2021.683594. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

