Lung Volume Calculation in Preclinical MicroCT: A Fast Geometrical Approach
- PMID: 35893082
- PMCID: PMC9330811
- DOI: 10.3390/jimaging8080204
Lung Volume Calculation in Preclinical MicroCT: A Fast Geometrical Approach
Abstract
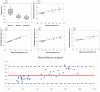
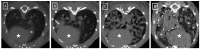
In this study, we present a time-efficient protocol for thoracic volume calculation as a proxy for total lung volume. We hypothesize that lung volume can be calculated indirectly from this thoracic volume. We compared the measured thoracic volume with manually segmented and automatically thresholded lung volumes, with manual segmentation as the gold standard. A linear regression formula was obtained and used for calculating the theoretical lung volume. This volume was compared with the gold standard volumes. In healthy animals, thoracic volume was 887.45 mm3, manually delineated lung volume 554.33 mm3 and thresholded aerated lung volume 495.38 mm3 on average. Theoretical lung volume was 554.30 mm3. Finally, the protocol was applied to three animal models of lung pathology (lung metastasis and transgenic primary lung tumor and fungal infection). In confirmed pathologic animals, thoracic volumes were: 893.20 mm3, 860.12 and 1027.28 mm3. Manually delineated volumes were 640.58, 503.91 and 882.42 mm3, respectively. Thresholded lung volumes were 315.92 mm3, 408.72 and 236 mm3, respectively. Theoretical lung volume resulted in 635.28, 524.30 and 863.10.42 mm3. No significant differences were observed between volumes. This confirmed the potential use of this protocol for lung volume calculation in pathologic models.
Keywords: lung; microCT; preclinical imaging; quantification; volume.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Velde G.V., De Langhe E., Poelmans J., Bruyndonckx P., D’Agostino E., Verbeken E., Bogaerts R., Lories R., Himmelreich U. Longitudinal in vivo microcomputed tomography of mouse lungs: No evidence for radiotoxicity. Am. J. Physiol. Cell. Mol. Physiol. 2015;309:L271–L279. doi: 10.1152/ajplung.00098.2015. - DOI - PMC - PubMed
-
- Çetinçakmak M.G., Göya C., Hamidi C., Tekbaş G., Abakay Ö., Batmaz I., Hattapoğlu S., Yavuz A., Bilici A. Quantitative volumetric assessment of pulmonary involvement in patients with systemic sclerosis. Quant. Imaging Med. Surg. 2016;6:50–56. doi: 10.3978/j.issn.2223-4292.2016.02.03. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

