Role of pH in Regulating Cancer Pyrimidine Synthesis
- PMID: 35893264
- PMCID: PMC9326563
- DOI: 10.3390/jox12030014
Role of pH in Regulating Cancer Pyrimidine Synthesis
Abstract
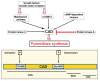
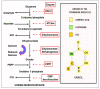
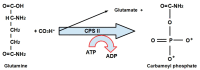
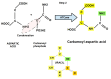
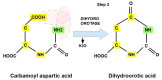
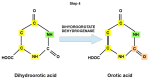
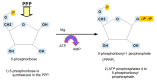
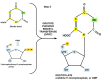
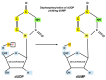
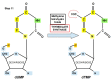
Replication is a fundamental aspect of cancer, and replication is about reproducing all the elements and structures that form a cell. Among them are DNA, RNA, enzymes, and coenzymes. All the DNA is doubled during each S (synthesis) cell cycle phase. This means that six billion nucleic acids must be synthesized in each cycle. Tumor growth, proliferation, and mutations all depend on this synthesis. Cancer cells require a constant supply of nucleotides and other macromolecules. For this reason, they must stimulate de novo nucleotide synthesis to support nucleic acid provision. When deregulated, de novo nucleic acid synthesis is controlled by oncogenes and tumor suppressor genes that enable increased synthesis and cell proliferation. Furthermore, cell duplication must be achieved swiftly (in a few hours) and in the midst of a nutrient-depleted and hypoxic environment. This also means that the enzymes participating in nucleic acid synthesis must work efficiently. pH is a critical factor in enzymatic efficiency and speed. This review will show that the enzymatic machinery working in nucleic acid synthesis requires a pH on the alkaline side in most cases. This coincides with many other pro-tumoral factors, such as the glycolytic phenotype, benefiting from an increased intracellular pH. An increased intracellular pH is a perfect milieu for high de novo nucleic acid production through optimal enzymatic performance.
Keywords: de novo nucleotide synthesis; intracellular alkalosis; pH deregulation; pyrimidine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ehrlich P. Über den jetzigen Stand der Chemotherapie. Ber. Dtsch. Chem. Ges. 1909;42:17–47. doi: 10.1002/cber.19090420105. - DOI
-
- Mukherjee S. In: Emperor of All Maladies: A Biography of Cancer. Mukherjee S., editor. Scribner; New York, NY, USA: 2010.
-
- Huggins C., Scott W.W., Hodges C.V. Studies on Prostatic Cancer. III. The Effects of Fever, of Desoxycorticosterone and of Estrogen on Clinical Patients with Metastatic Carcinoma of the Prostate1,2. J. Urol. 1941;46:997–1006. doi: 10.1016/S0022-5347(17)71004-X. - DOI
Publication types
LinkOut - more resources
Full Text Sources

