Prioritisation of Adverse Drug Events Leading to Hospital Admission and Occurring during Hospitalisation: A RAND Survey
- PMID: 35893345
- PMCID: PMC9332872
- DOI: 10.3390/jcm11154254
Prioritisation of Adverse Drug Events Leading to Hospital Admission and Occurring during Hospitalisation: A RAND Survey
Abstract
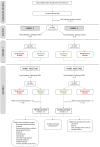
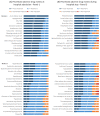
(1) Adverse drug events (ADEs) are a common cause of emergency department visits and occur frequently during hospitalisation. Instruments that facilitate the detection of the most relevant ADEs could lead to a more targeted and efficient use of limited resources in research and practice. (2) We conducted two consensus processes based on the RAND/UCLA appropriateness method, in order to prioritise ADEs leading to hospital admission (panel 1) and occurring during hospital stay (panel 2) for inclusion in future ADE measurement instruments. In each panel, the experts were asked to assess the "overall importance" of each ADE on a four-point Likert scale (1 = not important to 4 = very important). ADEs with a median rating of ≥3 without disagreement were defined as "prioritised". (3) The 13 experts in panel 1 prioritised 38 out of 65 ADEs, while the 12 experts in panel 2 prioritised 34 out of 63 ADEs. The highest rated events were acute kidney injury and hypoglycaemia (both panels), as well as Stevens-Johnson syndrome in panel 1 and rhabdomyolysis in panel 2. (4) The survey led to a set of ADEs for which there was consensus that they were of particular importance as presentations of acute medication-related harm, thereby providing a focus for further medication safety research and clinical practice.
Keywords: RAND survey; adverse drug events; consensus; drug-related side effects; medication safety; prioritisation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Laureau M., Vuillot O., Gourhant V., Perier D., Pinzani V., Lohan L., Faucanie M., Macioce V., Marin G., Giraud I., et al. Adverse Drug Events Detected by Clinical Pharmacists in an Emergency Department: A Prospective Monocentric Observational Study. J. Patient Saf. 2021;17:e1040–e1049. doi: 10.1097/PTS.0000000000000679. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

