Abdominal Pain in Inflammatory Bowel Diseases: A Clinical Challenge
- PMID: 35893357
- PMCID: PMC9331632
- DOI: 10.3390/jcm11154269
Abdominal Pain in Inflammatory Bowel Diseases: A Clinical Challenge
Abstract
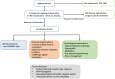
Up to 60% of inflammatory bowel disease (IBD) patients experience abdominal pain in their lifetime regardless of disease activity. Pain negatively affects different areas of daily life and particularly impacts the quality of life of IBD patients. This review provides a comprehensive overview of the multifactorial etiology implicated in the chronic abdominal pain of IBD patients including peripheral sensitization by inflammation, coexistent irritable bowel syndrome, visceral hypersensitivity, alteration of the brain-gut axis, and the multiple factors contributing to pain persistence. Despite the optimal management of intestinal inflammation, chronic abdominal pain can persist, and pharmacological and non-pharmacological approaches are necessary. Integrating psychological support in care models in IBD could decrease disease burden and health care costs. Consequently, a multidisciplinary approach similar to that used for other chronic pain conditions should be recommended.
Keywords: abdominal pain; inflammatory bowel disease; quality of life.
Conflict of interest statement
P.W. declares no conflict of interest. B.C. declares no conflict of interest. F.D. declares no conflict of interest. S.D. has served as a speaker, consultant, and advisory board member for Schering-Plough, AbbVie, Actelion, Alphawasserman, AstraZeneca, Cellerix, Cosmo Pharmaceuticals, Ferring, Genentech, Grunenthal, Johnson and Johnson, Millenium Takeda, MSD, Nikkiso Europe GmbH, Novo Nordisk, Nycomed, Pfizer, Pharmacosmos, UCB Pharma, and Vifor. L.P.-B. declares personal fees from Galapagos, AbbVie, Janssen, Genentech, Ferring, Tillots, Celltrion, Takeda, Pfizer, Index Pharmaceuticals, Sandoz, Celgene, Biogen, Samsung Bioepis, Inotrem, Allergan, MSD, Roche, Arena, Gilead, Amgen, BMS, Vifor, Norgine, Mylan, Lilly, Fresenius Kabi, OSE Immunotherapeutics, Enthera, Theravance, Pandion Therapeutics, Gossamer Bio, Viatris, Thermo Fisher; grants from Abbvie, MSD, Takeda, and Fresenius Kabi; stock options: CTMA.
Figures

References
-
- Khanna R., Zou G., D’Haens G., Feagan B.G., Sandborn W.J., Vandervoort M.K., Rolleri R.L., Bortey E., Paterson C., Forbes W.P., et al. A Retrospective Analysis: The Development of Patient Reported Outcome Measures for the Assessment of Crohn’s Disease Activity. Aliment. Pharmacol. Ther. 2015;41:77–86. doi: 10.1111/apt.13001. - DOI - PubMed
-
- Turner D., Ricciuto A., Lewis A., D’Amico F., Dhaliwal J., Griffiths A.M., Bettenworth D., Sandborn W.J., Sands B.E., Reinisch W., et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target Strategies in IBD. Gastroenterology. 2021;160:1570–1583. doi: 10.1053/j.gastro.2020.12.031. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

