Sex/Gender- and Age-Related Differences in β-Adrenergic Receptor Signaling in Cardiovascular Diseases
- PMID: 35893368
- PMCID: PMC9330499
- DOI: 10.3390/jcm11154280
Sex/Gender- and Age-Related Differences in β-Adrenergic Receptor Signaling in Cardiovascular Diseases
Abstract
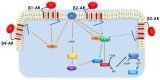
Sex differences in cardiovascular disease (CVD) are often recognized from experimental and clinical studies examining the prevalence, manifestations, and response to therapies. Compared to age-matched men, women tend to have reduced CV risk and a better prognosis in the premenopausal period. However, with menopause, this risk increases exponentially, surpassing that of men. Although several mechanisms have been provided, including sex hormones, an emerging role in these sex differences has been suggested for β-adrenergic receptor (β-AR) signaling. Importantly, β-ARs are the most important G protein-coupled receptors (GPCRs), expressed in almost all the cell types of the CV system, and involved in physiological and pathophysiological processes. Consistent with their role, for decades, βARs have been considered the first targets for rational drug design to fight CVDs. Of note, β-ARs are seemingly associated with different CV outcomes in females compared with males. In addition, even if there is a critical inverse correlation between β-AR responsiveness and aging, it has been reported that gender is crucially involved in this age-related effect. This review will discuss how β-ARs impact the CV risk and response to anti-CVD therapies, also concerning sex and age. Further, we will explore how estrogens impact β-AR signaling in women.
Keywords: G protein-coupled receptors; cardiovascular disease; sex differences; β-adrenergic receptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Age and sex mediated effects of estrogen and Β3-adrenergic receptor on cardiovascular pathophysiology.Exp Gerontol. 2024 Jun 1;190:112420. doi: 10.1016/j.exger.2024.112420. Epub 2024 Apr 8. Exp Gerontol. 2024. PMID: 38588751 Review.
-
Sex/Gender-Specific Imbalance in CVD: Could Physical Activity Help to Improve Clinical Outcome Targeting CVD Molecular Mechanisms in Women?Int J Mol Sci. 2020 Feb 21;21(4):1477. doi: 10.3390/ijms21041477. Int J Mol Sci. 2020. PMID: 32098263 Free PMC article. Review.
-
Sex-Gender Disparities in Cardiovascular Diseases: The Effects of Estrogen on eNOS, Lipid Profile, and NFATs During Catecholamine Stress.Front Cardiovasc Med. 2021 Feb 12;8:639946. doi: 10.3389/fcvm.2021.639946. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 33644139 Free PMC article. Review.
-
Age increases cardiac Galpha(i2) expression, resulting in enhanced coupling to G protein-coupled receptors.J Biol Chem. 2002 Aug 23;277(34):31257-62. doi: 10.1074/jbc.M203640200. Epub 2002 Jun 13. J Biol Chem. 2002. PMID: 12065589
-
Structure of β-adrenergic receptors.Methods Enzymol. 2013;520:117-51. doi: 10.1016/B978-0-12-391861-1.00006-X. Methods Enzymol. 2013. PMID: 23332698
Cited by
-
Gender and Renal Insufficiency: Opportunities for Their Therapeutic Management?Cells. 2022 Nov 29;11(23):3820. doi: 10.3390/cells11233820. Cells. 2022. PMID: 36497080 Free PMC article. Review.
-
Estrogen downregulates CD73/adenosine axis hyperactivity via adaptive modulation PI3K/Akt signaling to prevent myocarditis and arrhythmias during chronic catecholamines stress.Cell Commun Signal. 2023 Feb 23;21(1):41. doi: 10.1186/s12964-023-01052-0. Cell Commun Signal. 2023. PMID: 36823590 Free PMC article.
-
Hyperuricemia and Endothelial Function: Is It a Simple Association or Do Gender Differences Play a Role in This Binomial?Biomedicines. 2022 Nov 29;10(12):3067. doi: 10.3390/biomedicines10123067. Biomedicines. 2022. PMID: 36551823 Free PMC article. Review.
-
Sex-dependent influence of LMAN1 on allergen-induced airway hyperresponsiveness.J Immunol. 2025 Jun 15:vkaf126. doi: 10.1093/jimmun/vkaf126. Online ahead of print. J Immunol. 2025. PMID: 40517435
-
Stress and Obesity Signaling Converge on CREB Phosphorylation to Promote Pancreatic Cancer.Mol Cancer Res. 2025 Mar 3;23(3):236-249. doi: 10.1158/1541-7786.MCR-24-0785. Mol Cancer Res. 2025. PMID: 39642318
References
-
- Tsao C.W., Aday A.W., Almarzooq Z.I., Alonso A., Beaton A.Z., Bittencourt M.S., Boehme A.K., Buxton A.E., Carson A.P., Commodore-Mensah Y., et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

