Antibacterial Designs for Implantable Medical Devices: Evolutions and Challenges
- PMID: 35893454
- PMCID: PMC9326756
- DOI: 10.3390/jfb13030086
Antibacterial Designs for Implantable Medical Devices: Evolutions and Challenges
Abstract
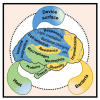
The uses of implantable medical devices are safer and more common since sterilization methods and techniques were established a century ago; however, device-associated infections (DAIs) are still frequent and becoming a leading complication as the number of medical device implantations keeps increasing. This urges the world to develop instructive prevention and treatment strategies for DAIs, boosting the studies on the design of antibacterial surfaces. Every year, studies associated with DAIs yield thousands of publications, which here are categorized into four groups, i.e., antibacterial surfaces with long-term efficacy, cell-selective capability, tailored responsiveness, and immune-instructive actions. These innovations are promising in advancing the solution to DAIs; whereas most of these are normally quite preliminary "proof of concept" studies lacking exact clinical scopes. To help identify the flaws of our current antibacterial designs, clinical features of DAIs are highlighted. These include unpredictable onset, site-specific incidence, and possibly involving multiple and resistant pathogenic strains. The key point we delivered is antibacterial designs should meet the specific requirements of the primary functions defined by the "intended use" of an implantable medical device. This review intends to help comprehend the complex relationship between the device, pathogens, and the host, and figure out future directions for improving the quality of antibacterial designs and promoting clinical translations.
Keywords: antibiotic resistance; antimicrobials; bacterial charging; biocompatibility; cell-selective surfaces; implantable antibacterial surfaces; polymicrobial infections; protein adsorption; surface modification; tissue integration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Andersen O.Z., Offermanns V., Sillassen M., Almtoft K.P., Andersen I.H., Sørensen S., Jeppesen C.S., Kraft D.C.E., Bøttiger J., Rasse M., et al. Accelerated bone ingrowth by local delivery of strontium from surface functionalized titanium implants. Biomaterials. 2013;34:5883–5890. doi: 10.1016/j.biomaterials.2013.04.031. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

