A Three-Dimensional Printed Polycaprolactone-Biphasic-Calcium-Phosphate Scaffold Combined with Adipose-Derived Stem Cells Cultured in Xenogeneic Serum-Free Media for the Treatment of Bone Defects
- PMID: 35893462
- PMCID: PMC9326540
- DOI: 10.3390/jfb13030093
A Three-Dimensional Printed Polycaprolactone-Biphasic-Calcium-Phosphate Scaffold Combined with Adipose-Derived Stem Cells Cultured in Xenogeneic Serum-Free Media for the Treatment of Bone Defects
Abstract
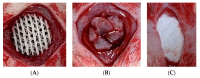
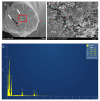
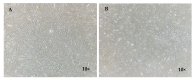
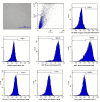
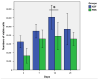
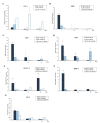
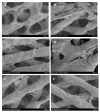
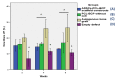
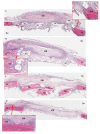
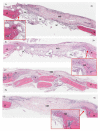
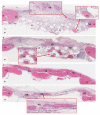
The efficacy of a three-dimensional printed polycaprolactone-biphasic-calcium-phosphate scaffold (PCL-BCP TDP scaffold) seeded with adipose-derived stem cells (ADSCs), which were cultured in xenogeneic serum-free media (XSFM) to enhance bone formation, was assessed in vitro and in animal models. The ADSCs were isolated from the buccal fat tissue of six patients using enzymatic digestion and the plastic adherence method. The proliferation and osteogenic differentiation of the cells cultured in XSFM when seeded on the scaffolds were assessed and compared with those of cells cultured in a medium containing fetal bovine serum (FBS). The cell-scaffold constructs were cultured in XSFM and were implanted into calvarial defects in thirty-six Wistar rats to assess new bone regeneration. The proliferation and osteogenic differentiation of the cells in the XSFM medium were notably better than that of the cells in the FBS medium. However, the efficacy of the constructs in enhancing new bone formation in the calvarial defects of rats was not statistically different to that achieved using the scaffolds alone. In conclusion, the PCL-BCP TDP scaffolds were biocompatible and suitable for use as an osteoconductive framework. The XSFM medium could support the proliferation and differentiation of ADSCs in vitro. However, the cell-scaffold constructs had no benefit in the enhancement of new bone formation in animal models.
Keywords: adipose; biphasic calcium phosphate; polycaprolactone; scaffold; stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Effects of three-dimensionally printed polycaprolactone/β-tricalcium phosphate scaffold on osteogenic differentiation of adipose tissue- and bone marrow-derived stem cells.Arch Craniofac Surg. 2018 Sep;19(3):181-189. doi: 10.7181/acfs.2018.01879. Epub 2018 Sep 20. Arch Craniofac Surg. 2018. PMID: 30282427 Free PMC article.
-
Effects of polycaprolactone-biphasic calcium phosphate scaffolds on enhancing growth and differentiation of osteoblasts.Biomed Mater Eng. 2018;29(2):159-176. doi: 10.3233/BME-171720. Biomed Mater Eng. 2018. PMID: 29457591
-
PCL/Col I-based magnetic nanocomposite scaffold provides an osteoinductive environment for ADSCs in osteogenic cues-free media conditions.Stem Cell Res Ther. 2022 Apr 4;13(1):143. doi: 10.1186/s13287-022-02816-0. Stem Cell Res Ther. 2022. PMID: 35379318 Free PMC article.
-
Evaluation of osteoconductive effect of polycaprolactone (PCL) scaffold treated with Aloe vera on adipose-derived mesenchymal stem cells (ADSCs).Am J Stem Cells. 2023 Oct 20;12(4):83-91. eCollection 2023. Am J Stem Cells. 2023. PMID: 38021455 Free PMC article.
-
Evaluation of the osteogenic potential of rat adipose-derived stem cells with different polycaprolactone/alginate-based nanofibrous scaffolds: an in vitro study.Stem Cell Investig. 2020 Aug 7;7:14. doi: 10.21037/sci-2020-015. eCollection 2020. Stem Cell Investig. 2020. PMID: 32964007 Free PMC article.
Cited by
-
IL-1b in the Secretomes of MSCs Seeded on Human Decellularized Allogeneic Bone Promotes Angiogenesis.Int J Mol Sci. 2022 Dec 4;23(23):15301. doi: 10.3390/ijms232315301. Int J Mol Sci. 2022. PMID: 36499629 Free PMC article.
-
Physical Characteristics and Biocompatibility of 3D-Printed Polylactic-Co-Glycolic Acid Membranes Used for Guided Bone Regeneration.J Funct Biomater. 2023 May 14;14(5):275. doi: 10.3390/jfb14050275. J Funct Biomater. 2023. PMID: 37233385 Free PMC article.
-
Enhanced In Vitro Biocompatible Polycaprolactone/Nano-Hydroxyapatite Scaffolds with Near-Field Direct-Writing Melt Electrospinning Technology.J Funct Biomater. 2022 Sep 23;13(4):161. doi: 10.3390/jfb13040161. J Funct Biomater. 2022. PMID: 36278630 Free PMC article.
References
-
- Thuaksuban N., Luntheng T., Monmaturapoj N. Physical characteristics and biocompatibility of the polycaprolactone-biphasic calcium phosphate scaffolds fabricated using the modified melt stretching and multilayer deposition. J. Biomater. Appl. 2016;30:1460–1472. doi: 10.1177/0885328216633890. - DOI - PubMed
-
- Wongsupa N., Nuntanaranont T., Kamolmattayakul S., Thuaksuban N. Assessment of bone regeneration of a tissue-engineered bone complex using human dental pulp stem cells/poly(epsilon-caprolactone)-biphasic calcium phosphate scaffold constructs in rabbit calvarial defects. J. Mater. Sci. Mater. Med. 2017;28:77. doi: 10.1007/s10856-017-5883-x. - DOI - PubMed
-
- Wongsupa N., Nuntanaranont T., Kamolmattayakul S., Thuaksuban N. Biological characteristic effects of human dental pulp stem cells on poly-epsilon-caprolactone-biphasic calcium phosphate fabricated scaffolds using modified melt stretching and multilayer deposition. J. Mater. Sci. Mater. Med. 2017;28:25. doi: 10.1007/s10856-016-5833-z. - DOI - PubMed
LinkOut - more resources
Full Text Sources

