Progress in Antiviral Fullerene Research
- PMID: 35893515
- PMCID: PMC9330071
- DOI: 10.3390/nano12152547
Progress in Antiviral Fullerene Research
Abstract
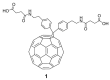
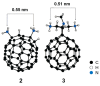
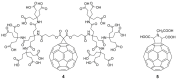
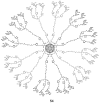
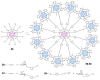
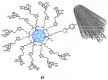
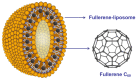
Unlike traditional small molecule drugs, fullerene is an all-carbon nanomolecule with a spherical cage structure. Fullerene exhibits high levels of antiviral activity, inhibiting virus replication in vitro and in vivo. In this review, we systematically summarize the latest research regarding the different types of fullerenes investigated in antiviral studies. We discuss the unique structural advantage of fullerenes, present diverse modification strategies based on the addition of various functional groups, assess the effect of structural differences on antiviral activity, and describe the possible antiviral mechanism. Finally, we discuss the prospective development of fullerenes as antiviral drugs.
Keywords: antivirus; fullerene; nanodrug; water-soluble fullerene derivatives.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


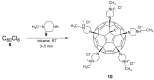
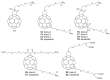
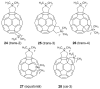
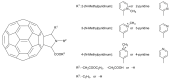
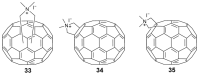

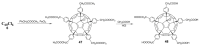

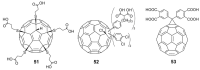
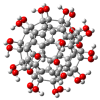

References
-
- Kroto H.W., Heath J.R., O’Brien S.C., Curl R.F., Smalley R.E. C60: Buckminsterfullerene. Nature. 1985;318:162–163. doi: 10.1038/318162a0. - DOI
-
- Krätschmer W., Lamb L.D., Fostiropoulos K., Huffman D.R. Solid C60: A new form of carbon. Nature. 1990;347:354–358. doi: 10.1038/347354a0. - DOI
-
- Zhang H.-G., Zhuo Y.-Q., Zhang X.-M., Zhang L., Xu P.-Y., Tian H.-R., Lin S.-C., Zhang Q., Xie S.-Y., Zheng L.-S. Synthesis of Fullerenes from a Nonaromatic Chloroform through a Newly Developed Ultrahigh-Temperature Flash Vacuum Pyrolysis Apparatus. Nanomaterials. 2021;11:3033. doi: 10.3390/nano11113033. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

