Visible-Light-Active Black TiO2 Nanoparticles with Efficient Photocatalytic Performance for Degradation of Pharmaceuticals
- PMID: 35893534
- PMCID: PMC9330099
- DOI: 10.3390/nano12152563
Visible-Light-Active Black TiO2 Nanoparticles with Efficient Photocatalytic Performance for Degradation of Pharmaceuticals
Abstract
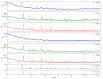
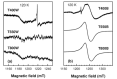
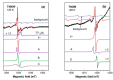
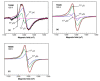
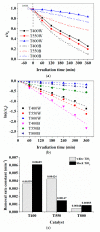
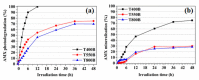
Special attention has recently been paid to surface-defective titanium dioxide and black TiO2 with advanced optical, electrical, and photocatalytic properties. Synthesis of these materials for photodegradation and mineralization of persistent organic pollutants in water, especially under visible radiation, presents interest from scientific and application points of view. Chemical reduction by heating a TiO2 and NaBH4 mixture at 350 °C successfully introduced Ti3+ defects and oxygen vacancies at the surface of TiO2, with an increase in the photocatalytic degradation of amoxicillin-an antibiotic that is present in wastewater due to its intense use in human and animal medicine. Three TiO2 samples were prepared at different annealing temperatures to control the ratio between anatase and rutile and were subjected to chemical reduction. Electron paramagnetic resonance investigations showed that the formation of surface Ti3+ defects in a high concentration occurred mainly in the anatase sample annealed at 400 °C, contributing to the bandgap reduction from 3.32 eV to 2.92 eV. The reduced band gap enhances visible light absorption and the efficiency of photocatalysis. The nanoparticles of ~90 m2/g specific surface area and 12 nm average size exhibit ~100% efficiency in the degradation of amoxicillin under simulated solar irradiation compared with pristine TiO2. Mineralization of amoxicillin and by-products was over 75% after 48 h irradiation for the anatase sample, where the Ti3+ defects were present in a higher concentration at the catalyst's surface.
Keywords: Ti3+ states; amoxicillin; black TiO2; chemical reduction; defective TiO2; photocatalysis; wastewater treatment.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Hasija V., Raizada P., Hosseini-Bandegharaei A., Thakur V.K., Van Le Q., Nguyen V.H., Singh P. A Strategy to Develop Efficient Ag3PO4-based Photocatalytic Materials Toward Water Splitting: Perspectives and Challenges. ChemCatChem. 2021;13:2965–2987. doi: 10.1002/cctc.202100135. - DOI
-
- Kumar M.P., Jagannathan R., Ravichandran S. Photoelectrochemical System for Unassisted High-Efficiency Water-Splitting Reactions Using N-Doped TiO2 Nanotubes. Energy Fuels. 2020;34:9030–9036. doi: 10.1021/acs.energyfuels.0c00634. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

