Differential Antivenom and Small-Molecule Inhibition of Novel Coagulotoxic Variations in Atropoides, Cerrophidion, Metlapilcoatlus, and Porthidium American Viperid Snake Venoms
- PMID: 35893753
- PMCID: PMC9332056
- DOI: 10.3390/toxins14080511
Differential Antivenom and Small-Molecule Inhibition of Novel Coagulotoxic Variations in Atropoides, Cerrophidion, Metlapilcoatlus, and Porthidium American Viperid Snake Venoms
Abstract
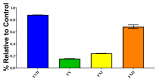
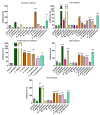
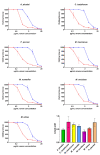
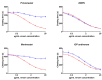
Within Neotropical pit-vipers, the Mexican/Central-American clade consisting of Atropoides, Cerrophidion, Metlapilcoatlus, and Porthidium is a wide-ranging, morphologically and ecologically diverse group of snakes. Despite their prevalence, little is known of the functional aspects of their venoms. This study aimed to fill the knowledge gap regarding coagulotoxic effects and to examine the potential of different therapeutic approaches. As a general trait, the venoms were shown to be anticoagulant but were underpinned by diverse biochemical actions. Pseudo-procoagulant activity (i.e., thrombin-like), characterized by the direct cleavage of fibrinogen to form weak fibrin clots, was evident for Atropoides picadoi, Cerrophidiontzotzilorum, Metlapilcoatlus mexicanus, M. nummifer, M. occiduus, M. olmec, and Porthidium porrasi. In contrast, other venoms cleaved fibrinogen in a destructive (non-clotting) manner, with C. godmani and C. wilsoni being the most potent. In addition to actions on fibrinogen, clotting enzymes were also inhibited. FXa was only weakly inhibited by most species, but Cerrophidion godmani and C. wilsoni were extremely strong in their inhibitory action. Other clotting enzymes were more widely inhibited by diverse species spanning the full taxonomical range, but in each case, there were species that had these traits notably amplified relatively to the others. C. godmani and C. wilsoni were the most potent amongst those that inhibited the formation of the prothrombinase complex and were also amongst the most potent inhibitors of Factor XIa. While most species displayed only low levels of thrombin inhibition, Porthidium dunni potently inhibited this clotting factor. The regional polyvalent antivenom produced by Instituto Picado Clodomiro was tested and was shown to be effective against the diverse anticoagulant pathophysiological effects. In contrast to the anticoagulant activities of the other species, Porthidium volcanicum was uniquely procoagulant through the activation of Factor VII and Factor XII. This viperid species is the first snake outside of the Oxyuranus/Pseudonaja elapid snake clade to be shown to activate FVII and the first snake venom of any kind to activate FXII. Interestingly, while small-molecule metalloprotease inhibitors prinomastat and marimastat demonstrated the ability to prevent the procoagulant toxicity of P. volcanicum, neither ICP antivenom nor inhibitor DMPS showed this effect. The extreme variation among the snakes here studied underscores how venom is a dynamic trait and how this can shape clinical outcomes and influence evolving treatment strategies.
Keywords: anticoagulant; antivenom; procoagulant; pseudo-procoagulant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Neri-Castro E., Bénard-Valle M., de León J.L., Boyer L., Alagón A. Handbook of Venoms and Toxins of Reptiles. CRC Press; Boca Raton, FL, USA: 2021. Envenomations by Reptiles in Mexico; pp. 529–542.
-
- Gutiérrez J. Handbook of Venoms and Toxins of Reptiles. CRC Press; Boca Raton, FL, USA: 2009. Snakebite envenomation in Central America; pp. 491–507.
-
- Boyer L., Alagón A., Fry B.G., Jackson T.N.W., Sunagar K., Chippaux J.P. Signs, Symptoms and Treatment of Envenomation. In: Fry B.G., editor. Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery. Oxford University Press; New York, NY, USA: 2015. pp. 32–60.
-
- Mackessy S.P. Handbook of Venoms and Toxins of Reptiles. CRC Press; Boca Raton, FL, USA: 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

