Itraconazole Nanosuspensions via Dual Centrifugation Media Milling: Impact of Formulation and Process Parameters on Particle Size and Solid-State Conversion as Well as Storage Stability
- PMID: 35893783
- PMCID: PMC9332252
- DOI: 10.3390/pharmaceutics14081528
Itraconazole Nanosuspensions via Dual Centrifugation Media Milling: Impact of Formulation and Process Parameters on Particle Size and Solid-State Conversion as Well as Storage Stability
Abstract
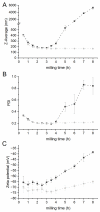
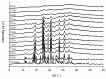
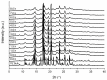
Nanocrystal suspensions proved to be a potent enabling principle for biopharmaceutics classification system class II drugs with dissolution limited bioavailability. In the example of itraconazole (ITZ) as a model drug combined with electrosteric stabilization using hydroxypropyl cellulose (HPC-SL), sodium dodecyl sulfate (SDS) and polysorbate 80 (PS80), the impacts of formulation and process parameters of a dual centrifugal mill on material attributes such as particle size, zeta potential, particle morphology, storage stability and especially solid-state characteristics were evaluated. A minimal concentration of 0.9% (w/w) HPC-SL, 0.14% (w/w) SDS and 0.07% (w/w) PS80 was necessary for sufficient nanoparticle stabilization. Despite the minor effect of PS80, its presence was beneficial for electrosteric stabilization. Choosing lower stabilizer concentrations resulted in a pronounced increase in particle size due to agglomeration, which was confirmed by SEM imaging and a decrease in zeta potential in combination with an amorphization of the particles. Milling temperature had no significant impact on the particle size, whereas milling speed and the size of the milling beads used were found to have a strong impact on the critical material attributes such as particle size and polydispersity index. The smallest particle sizes could be obtained by using the smallest milling bead size. However, the smallest obtainable particle size could only be achieved by using two-fold stabilizer concentrations, as smaller particles exhibit a larger specific surface area.
Keywords: agglomeration; amorphization; dual centrifugation; itraconazole; media milling; nanocrystals; nanosuspension.
Conflict of interest statement
Ann-Cathrin Willmann, Kai Berkenfeld and Herbert Wachtel are at the time of submission employees at Boehringer Ingelheim Pharma GmbH & Co. KG. Georg Boeck is at the time of submission an employee of Boehringer Ingelheim RCV GmbH & Co. KG. The authors declare no further conflict of interest.
Figures




References
-
- Noyes A., Whitney W.R. The Rate of Solution of Solid Substances in Their Own Solutions. J. Am. Chem. Soc. 1897;19:930–934. doi: 10.1021/ja02086a003. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

