Localized Controlled Release of Kynurenic Acid Encapsulated in Synthetic Polymer Reduces Implant-Induced Dermal Fibrosis
- PMID: 35893802
- PMCID: PMC9331703
- DOI: 10.3390/pharmaceutics14081546
Localized Controlled Release of Kynurenic Acid Encapsulated in Synthetic Polymer Reduces Implant-Induced Dermal Fibrosis
Abstract
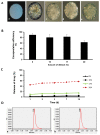
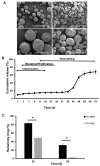
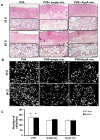
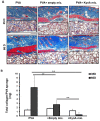
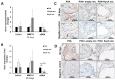
Excessive fibrosis following surgical procedures is a challenging condition with serious consequences and no effective preventive or therapeutic option. Our group has previously shown the anti-fibrotic effect of kynurenic acid (KynA) in vitro and as topical cream formulations or nanofiber dressings in open wounds. Here, we hypothesized that the implantation of a controlled release drug delivery system loaded with KynA in a wound bed can prevent fibrosis in a closed wound. Poly (lactic-co-glycolic acid) (PLGA), and a diblock copolymer, methoxy polyethylene glycol-block-poly (D, L-lactide) (MePEG-b-PDLLA), were used for the fabrication of microspheres which were evaluated for their characteristics, encapsulation efficiency, in vitro release profile, and in vivo efficacy for reduction of fibrosis. The optimized formulation exhibited high encapsulation efficiency (>80%), low initial burst release (~10%), and a delayed, gradual release of KynA. In vivo evaluation of the fabricated microspheres in the PVA model of wound healing revealed that KynA microspheres effectively reduced collagen deposition inside and around PVA sponges and α-smooth muscle actin expression after 66 days. Our results showed that KynA can be efficiently encapsulated in PLGA microspheres and its controlled release in vivo reduces fibrotic tissue formation, suggesting a novel therapeutic option for the prevention or treatment of post-surgical fibrosis.
Keywords: PLGA; fibrosis; kynurenic acid; microsphere.
Conflict of interest statement
Aziz Ghahary holds patent on kynurenic acid.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

