Micromolding of Amphotericin-B-Loaded Methoxyethylene-Maleic Anhydride Copolymer Microneedles
- PMID: 35893806
- PMCID: PMC9331399
- DOI: 10.3390/pharmaceutics14081551
Micromolding of Amphotericin-B-Loaded Methoxyethylene-Maleic Anhydride Copolymer Microneedles
Abstract
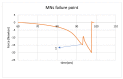
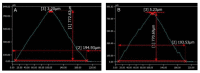
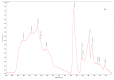
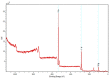
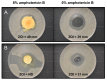
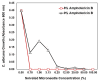
Biocompatible and biodegradable materials have been used for fabricating polymeric microneedles to deliver therapeutic drug molecules through the skin. Microneedles have advantages over other drug delivery methods, such as low manufacturing cost, controlled drug release, and the reduction or absence of pain. The study examined the delivery of amphotericin B, an antifungal agent, using microneedles that were fabricated using a micromolding technique. The microneedle matrix was made from GantrezTM AN-119 BF, a benzene-free methyl vinyl ether/maleic anhydride copolymer. The GantrezTM AN-119 BF was mixed with water; after water evaporation, the polymer exhibited sufficient strength for microneedle fabrication. Molds cured at room temperature remained sharp and straight. SEM images showed straight and sharp needle tips; a confocal microscope was used to determine the height and tip diameter for the microneedles. Nanoindentation was used to obtain the hardness and Young's modulus values of the polymer. Load-displacement testing was used to assess the failure force of the needles under compressive loading. These two mechanical tests confirmed the mechanical properties of the needles. In vitro studies validated the presence of amphotericin B in the needles and the antifungal properties of the needles. Amphotericin B GantrezTM microneedles fabricated in this study showed appropriate characteristics for clinical translation in terms of mechanical properties, sharpness, and antifungal properties.
Keywords: amphotericin B; fungus; microneedles; transdermal drug delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Gittard S.D., Narayan R.J. Toxicology of the Skin. CRC Press; Boca Raton, FL, USA: 2010. Applications of Microneedle Technology to Transdermal Drug Delivery; pp. 315–330.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

