Comparative Preclinical Study of Lidocaine and Mepivacaine in Resilient Hyaluronic Acid Fillers
- PMID: 35893810
- PMCID: PMC9329866
- DOI: 10.3390/pharmaceutics14081553
Comparative Preclinical Study of Lidocaine and Mepivacaine in Resilient Hyaluronic Acid Fillers
Abstract
Background: Hyaluronic acid-based filler injections are now well-established aesthetic procedures for the correction of skin tissue defects and volume loss. Filler injections are becoming increasingly popular, with a growing number of injections performed each year. Although classified as a minimally invasive procedure, the introduction of a needle or a canula may remain painful for the patient. A major improvement was achieved with the incorporation of local anesthetics into the formulation for pain relief.
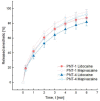
Methods: In this study, two well-known anesthetics, lidocaine and mepivacaine, were systematically compared to assess their influence on filler mechanical and biological features. The impact of each anesthetic was monitored in terms of gel rheological properties, stability, durability, and degradation. The release profiles of each anesthetic were also recorded. Finally, the pharmacokinetics of each anesthetic in rats were assessed.
Results: For all the rheological and biological experiments performed, lidocaine and mepivacaine influences were comparable. The addition of either anesthetic into a soft-tissue filler showed no significant modifications of the stability, durability, and degradability of the gel, with similar release profiles and pharmacokinetics at an equivalent concentration.
Conclusions: Substituting lidocaine with mepivacaine does not impact the properties of the gels, and thus both can be equally incorporated as anesthetics in soft-tissue fillers.
Keywords: anesthetics; hyaluronic acid; lidocaine; mepivacaine; pharmacokinetics; release; soft-tissue fillers.
Conflict of interest statement
All authors are employees of Teoxane SA.
Figures



References
-
- Royo de la Torre J., Moreno-Moraga J., Isarría M.J., Muñoz E., Cruz I., Pérez G., Cornejo P. The evaluation of hyaluronic acid, with and without lidocaine, in the filling of nasolabial folds as measured by ultrastructural changes and pain management. J. Drugs Dermatol. 2013;12:e46–e52. - PubMed
-
- Tetzlaff J.E. Cousins and Bridenbaugh’s Neural Blockade in Clinical Anesthesia and Pain Medicine. Mayo Clin. Proc. 2010;85:e51. doi: 10.4065/mcp.2010.0230. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

