Ciprofloxacin-Loaded Zein/Hyaluronic Acid Nanoparticles for Ocular Mucosa Delivery
- PMID: 35893813
- PMCID: PMC9332751
- DOI: 10.3390/pharmaceutics14081557
Ciprofloxacin-Loaded Zein/Hyaluronic Acid Nanoparticles for Ocular Mucosa Delivery
Abstract
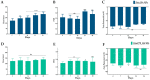
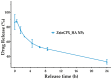
Bacterial conjunctivitis is a worldwide problem that, if untreated, can lead to severe complications, such as visual impairment and blindness. Topical administration of ciprofloxacin is one of the most common treatments for this infection; however, topical therapeutic delivery to the eye is quite challenging. To tackle this, nanomedicine presents several advantages compared to conventional ophthalmic dosage forms. Herein, the flash nanoprecipitation technique was applied to produce zein and hyaluronic acid nanoparticles loaded with ciprofloxacin (ZeinCPX_HA NPs). ZeinCPX_HA NPs exhibited a hydrodynamic diameter of <200 nm and polydispersity index of <0.3, suitable for ocular drug delivery. In addition, the freeze-drying of the nanoparticles was achieved by using mannitol as a cryoprotectant, allowing their resuspension in water without modifying the physicochemical properties. Moreover, the biocompatibility of nanoparticles was confirmed by in vitro assays. Furthermore, a high encapsulation efficiency was achieved, and a release profile with an initial burst was followed by a prolonged release of ciprofloxacin up to 24 h. Overall, the obtained results suggest ZeinCPX_HA NPs as an alternative to the common topical dosage forms available on the market to treat conjunctivitis.
Keywords: ciprofloxacin; conjunctivitis; flash nanoprecipitation; hyaluronic acid; nanoparticles; zein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Haq A., Wardak H., Kraski N. Infective Conjunctivitis—Its Pathogenesis, Management and Complications. In: Chaudhry I.A., editor. Common Eye Infections. IntechOpen; London, UK: 2013. - DOI
-
- Pippin M.M., Le J.K. Bacterial Conjunctivitis. [(accessed on 18 June 2022)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK546683/
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

