A Large Cluster of New Onset Autoimmune Myositis in the Yorkshire Region Following SARS-CoV-2 Vaccination
- PMID: 35893834
- PMCID: PMC9331977
- DOI: 10.3390/vaccines10081184
A Large Cluster of New Onset Autoimmune Myositis in the Yorkshire Region Following SARS-CoV-2 Vaccination
Abstract
Background: The novel SARS-CoV-2 vaccines partially exploit intrinsic DNA or RNA adjuvanticity, with dysregulation in the metabolism of both these nucleic acids independently linked to triggering experimental autoimmune diseases, including lupus and myositis.
Methods: Herein, we present 15 new onset autoimmune myositis temporally associated with SARS-CoV-2 RNA or DNA-based vaccines that occurred between February 2021 and April 2022. Musculoskeletal, pulmonary, cutaneous and cardiac manifestations, laboratory and imaging data were collected.
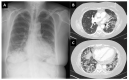
Results: In total, 15 cases of new onset myositis (11 polymyositis/necrotizing/overlap myositis; 4 dermatomyositis) were identified in the Yorkshire region of approximately 5.6 million people, between February 2021 and April 2022 (10 females/5 men; mean age was 66.1 years; range 37-83). New onset disease occurred after first vaccination (5 cases), second vaccination (7 cases) or after the third dose (3 cases), which was often a different vaccine. Of the cases, 6 had systemic complications including skin (3 cases), lung (3 cases), heart (2 cases) and 10/15 had myositis associated autoantibodies. All but 1 case had good therapy responses. Adverse event following immunization (AEFI) could not be explained based on the underlying disease/co-morbidities.
Conclusion: Compared with our usual regional Rheumatology clinical experience, a surprisingly large number of new onset myositis cases presented during the period of observation. Given that antigen release inevitably follows muscle injury and given the role of nucleic acid adjuvanticity in autoimmunity and muscle disease, further longitudinal studies are required to explore potential links between novel coronavirus vaccines and myositis in comparison with more traditional vaccine methods.
Keywords: COVID vaccination; adjuvanticity; myositis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. - DOI - PMC - PubMed
-
- Ella R., Vadrevu K.M., Jogdand H., Prasad S., Reddy S., Sarangi V., Ganneru B., Sapkal G., Yadav P., Abraham P., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: A double-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021;21:637–646. doi: 10.1016/S1473-3099(20)30942-7. - DOI - PMC - PubMed
-
- Ramasamy M.N., Minassian A.M., Ewer K.J. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

