Vitamin D and Secondary Hyperparathyroidism in Chronic Kidney Disease: A Critical Appraisal of the Past, Present, and the Future
- PMID: 35893866
- PMCID: PMC9330693
- DOI: 10.3390/nu14153009
Vitamin D and Secondary Hyperparathyroidism in Chronic Kidney Disease: A Critical Appraisal of the Past, Present, and the Future
Abstract
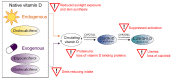
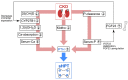
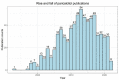
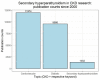
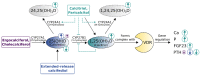
The association between vitamin D deficiency and especially critical shortage of active vitamin D (1,25-dihydroxyvitamin D, calcitriol) with the development of secondary hyperparathyroidism (sHPT) is a well-known fact in patients with chronic kidney disease (CKD). The association between sHPT and important clinical outcomes, such as kidney disease progression, fractures, cardiovascular events, and mortality, has turned the prevention and the control of HPT into a core issue of patients with CKD and on dialysis. However, vitamin D therapy entails the risk of unwanted side effects, such as hypercalcemia and hyperphosphatemia. This review summarizes the developments of vitamin D therapies in CKD patients of the last decades, from calcitriol substitution to extended-release calcifediol. In view of the study situation for vitamin D insufficiency and sHPT in CKD patients, we conclude that the nephrology community has to solve three core issues: (1) What is the optimal parathyroid hormone (PTH) target level for CKD and dialysis patients? (2) What is the optimal vitamin D level to support optimal PTH titration? (3) How can sHPT treatment support reduction in the occurrence of hard renal and cardiovascular events in CKD and dialysis patients?
Keywords: chronic kidney disease; chronic kidney disease–mineral and bone disorder; parathyroid hormone; secondary hyperparathyroidism; vitamin D; vitamin D insufficiency.
Conflict of interest statement
Markus Ketteler has received lecture fees and consulting honoraria from Amgen, Kyowa Kirin, Ono Pharmaceuticals, Vifor Fresenius Medical Care Renal Pharma and Vifor Pharma. Vincent Brandenburg has received lectures fees and consulting honoraria from Amgen Vifor Fresenius Medical Care Renal Pharma, Vifor Pharma, AstraZeneca, and Pfizer.
Figures
References
-
- Levey A.S., Eckardt K.-U., Dorman N.M., Christiansen S.L., Hoorn E.J., Ingelfinger J.R., Inker L.A., Levin A., Mehrotra R., Palevsky P.M., et al. Nomenclature for kidney function and disease: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020;97:1117–1129. doi: 10.1016/j.kint.2020.02.010. - DOI - PubMed
-
- de Boer I.H., Caramori M.L., Chan J.C., Heerspink H.J., Hurst C., Khunti K., Rossing P. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020;98:S1–S116. doi: 10.1016/j.kint.2020.06.019. - DOI - PubMed
-
- Isakova T., Cai X., Lee J., Mehta R., Zhang X., Yang W., Nessel L., Anderson A.H., Lo J., Porter A., et al. Longitudinal Evolution of Markers of Mineral Metabolism in Patients With CKD: The Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2020;75:235–244. doi: 10.1053/j.ajkd.2019.07.022. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

