Synergistic Effect of β-Lapachone and Aminooxyacetic Acid on Central Metabolism in Breast Cancer
- PMID: 35893874
- PMCID: PMC9331106
- DOI: 10.3390/nu14153020
Synergistic Effect of β-Lapachone and Aminooxyacetic Acid on Central Metabolism in Breast Cancer
Abstract
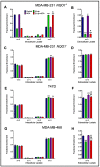
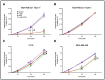
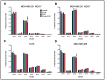
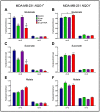
The compound β-lapachone, a naturally derived naphthoquinone, has been utilized as a potent medicinal nutrient to improve health. Over the last twelve years, numerous reports have demonstrated distinct associations of β-lapachone and NAD(P)H: quinone oxidoreductase 1 (NQO1) protein in the amelioration of various diseases. Comprehensive research of NQO1 bioactivity has clearly confirmed the tumoricidal effects of β-lapachone action through NAD+-keresis, in which severe DNA damage from reactive oxygen species (ROS) production triggers a poly-ADP-ribose polymerase-I (PARP1) hyperactivation cascade, culminating in NAD+/ATP depletion. Here, we report a novel combination strategy with aminooxyacetic acid (AOA), an aspartate aminotransferase inhibitor that blocks the malate-aspartate shuttle (MAS) and synergistically enhances the efficacy of β-lapachone metabolic perturbation in NQO1+ breast cancer. We evaluated metabolic turnover in MDA-MB-231 NQO1+, MDA-MB-231 NQO1-, MDA-MB-468, and T47D cancer cells by measuring the isotopic labeling of metabolites from a [U-13C]glucose tracer. We show that β-lapachone treatment significantly hampers lactate secretion by ~85% in NQO1+ cells. Our data demonstrate that combinatorial treatment decreases citrate, glutamate, and succinate enrichment by ~14%, ~50%, and ~65%, respectively. Differences in citrate, glutamate, and succinate fractional enrichments indicate synergistic effects on central metabolism based on the coefficient of drug interaction. Metabolic modeling suggests that increased glutamine anaplerosis is protective in the case of MAS inhibition.
Keywords: GC-MS; biogenic; cancer; isotopologue; metabolism; synergy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Kim J.H., Lee S.M., Myung C.H., Lee K.R., Hyun S.M., Lee J.E., Park Y.S., Jeon S.R., Park J.I., Chang S.E., et al. Melanogenesis Inhibition of β-Lapachone, a Natural Product from Tabebuia Avellanedae, with Effective in Vivo Lightening Potency. Arch. Dermatol. Res. 2015;307:229–238. doi: 10.1007/s00403-015-1543-5. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

