Ginsenoside Rh4 Suppresses Metastasis of Esophageal Cancer and Expression of c-Myc via Targeting the Wnt/β-Catenin Signaling Pathway
- PMID: 35893895
- PMCID: PMC9331240
- DOI: 10.3390/nu14153042
Ginsenoside Rh4 Suppresses Metastasis of Esophageal Cancer and Expression of c-Myc via Targeting the Wnt/β-Catenin Signaling Pathway
Abstract
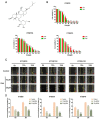
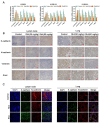
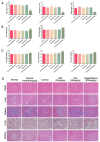
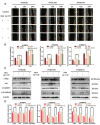
The metastasis of esophageal squamous cell carcinoma (ESCC) is a leading cause of death worldwide, however, it has a poor prognosis. Ginsenoside Rh4 is a rare saponin that has been shown to have potential antitumor effectiveness in ESCC. However, the utility of Rh4 in ESCC metastasis and its undiscovered mode of action has not yet been explored. In this study, we found that Rh4 could inhibit ESCC metastasis by regulating the Wnt/β-catenin signaling pathway and the level of c-Myc, which is an important transcription factor in cancer. In in vitro experiments, Rh4 could inhibit the migration and invasion of ESCC cells without affecting cell viability. In in vivo experiments, Rh4 restrained ESCC metastasis to the lymph nodes and lungs via the suppression of epithelial-mesenchymal transition (EMT). The Wnt agonist HLY78 promoted EMT and migration of ESCC cells, whereas treatment of Rh4 can attenuate the promotion effect of HLY78. The siRNA knocking out c-Myc can also significantly reduce the expression of EMT-related marker proteins. This study illustrates a new concept for further research on the mechanism of Rh4 in ESCC.
Keywords: ESCC; Wnt/β-catenin; c-Myc; ginsenoside Rh4; metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

