Hallmarks of neurodegenerative disease: A systems pharmacology perspective
- PMID: 35894182
- PMCID: PMC9662204
- DOI: 10.1002/psp4.12852
Hallmarks of neurodegenerative disease: A systems pharmacology perspective
Abstract
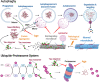
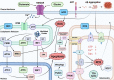
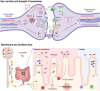
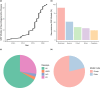
Age-related central neurodegenerative diseases, such as Alzheimer's and Parkinson's disease, are a rising public health concern and have been plagued by repeated drug development failures. The complex nature and poor mechanistic understanding of the etiology of neurodegenerative diseases has hindered the discovery and development of effective disease-modifying therapeutics. Quantitative systems pharmacology models of neurodegeneration diseases may be useful tools to enhance the understanding of pharmacological intervention strategies and to reduce drug attrition rates. Due to the similarities in pathophysiological mechanisms across neurodegenerative diseases, especially at the cellular and molecular levels, we envision the possibility of structural components that are conserved across models of neurodegenerative diseases. Conserved structural submodels can be viewed as building blocks that are pieced together alongside unique disease components to construct quantitative systems pharmacology (QSP) models of neurodegenerative diseases. Model parameterization would likely be different between the different types of neurodegenerative diseases as well as individual patients. Formulating our mechanistic understanding of neurodegenerative pathophysiology as a mathematical model could aid in the identification and prioritization of drug targets and combinatorial treatment strategies, evaluate the role of patient characteristics on disease progression and therapeutic response, and serve as a central repository of knowledge. Here, we provide a background on neurodegenerative diseases, highlight hallmarks of neurodegeneration, and summarize previous QSP models of neurodegenerative diseases.
© 2022 The Authors. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures


References
-
- Kaushik S, Cuervo AM. Proteostasis and aging. Nat Med. 2015;21:1406‐1415. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353‐356. - PubMed
-
- Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205‐214. - PubMed
-
- Hetz C, Saxena S. ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol. 2017;13:477‐491. - PubMed
-
- Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353:777‐783. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

