Association of Renin Angiotensin Aldosterone System Inhibitors and Outcomes of Hospitalized Patients With COVID-19
- PMID: 35894609
- PMCID: PMC9469914
- DOI: 10.1097/CCM.0000000000005627
Association of Renin Angiotensin Aldosterone System Inhibitors and Outcomes of Hospitalized Patients With COVID-19
Abstract
Objectives: To determine the association of prior use of renin-angiotensin-aldosterone system inhibitors (RAASIs) with mortality and outcomes in hospitalized patients with COVID-19.
Design: Retrospective observational study.
Setting: Multicenter, international COVID-19 registry.
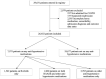
Subjects: Adult hospitalized COVID-19 patients on antihypertensive agents (AHAs) prior to admission, admitted from March 31, 2020, to March 10, 2021.
Interventions: None.
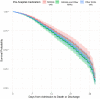
Measurements and main results: Data were compared between three groups: patients on RAASIs only, other AHAs only, and those on both medications. Multivariable logistic and linear regressions were performed after controlling for prehospitalization characteristics to estimate the effect of RAASIs on mortality and other outcomes during hospitalization. Of 26,652 patients, 7,975 patients were on AHAs prior to hospitalization. Of these, 1,542 patients (19.3%) were on RAASIs only, 3,765 patients (47.2%) were on other AHAs only, and 2,668 (33.5%) patients were on both medications. Compared with those taking other AHAs only, patients on RAASIs only were younger (mean age 63.3 vs 66.9 yr; p < 0.0001), more often male (58.2% vs 52.4%; p = 0.0001) and more often White (55.1% vs 47.2%; p < 0.0001). After adjusting for age, gender, race, location, and comorbidities, patients on combination of RAASIs and other AHAs had higher in-hospital mortality than those on RAASIs only (odds ratio [OR] = 1.28; 95% CI [1.19-1.38]; p < 0.0001) and higher mortality than those on other AHAs only (OR = 1.09; 95% CI [1.03-1.15]; p = 0.0017). Patients on RAASIs only had lower mortality than those on other AHAs only (OR = 0.87; 95% CI [0.81-0.94]; p = 0.0003). Patients on ACEIs only had higher mortality compared with those on ARBs only (OR = 1.37; 95% CI [1.20-1.56]; p < 0.0001).
Conclusions: Among patients hospitalized for COVID-19 who were taking AHAs, prior use of a combination of RAASIs and other AHAs was associated with higher in-hospital mortality than the use of RAASIs alone. When compared with ARBs, ACEIs were associated with significantly higher mortality in hospitalized COVID-19 patients.
Trial registration: ClinicalTrials.gov NCT04323787.
Copyright © 2022 by the Society of Critical Care Medicine and Wolters Kluwer Health, Inc. All Rights Reserved.
Conflict of interest statement
Dr. Perkins received funding from TelmedIQ. Dr. Kaufman’s institution received funding from the Society of Critical Care Medicine, and she received funding from consulting for Mendaera. Dr. Christie’s institution received funding from Navicent Health Foundation. Ms. Boman, Drs. Kumar’s, Walkey’s, and Kashyap’s institutions received funding from the Gordon and Betty Moore Foundation. Ms. Boman, Drs. Kumar’s, and Kashyap’s institutions received funding from Janssen R & D. Dr. Aston’s institution received funding from the National Institutes of Health. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures
References
-
- Furuhashi M, Moniwa N, Mita T, et al. : Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens 2015; 28:15–21 - PubMed

