3D Reconstruction of a Unitary Posterior Eye by Converging Optically Corrected Optical Coherence and Magnetic Resonance Tomography Images via 3D CAD
- PMID: 35895054
- PMCID: PMC9344223
- DOI: 10.1167/tvst.11.7.24
3D Reconstruction of a Unitary Posterior Eye by Converging Optically Corrected Optical Coherence and Magnetic Resonance Tomography Images via 3D CAD
Abstract
Purpose: In acquiring images of the posterior eye, magnetic resonance imaging (MRI) provides low spatial resolution of the overall shape of the eye while optical coherence tomography (OCT) offers high spatial resolution of the limited range. Through the merger of the two devices, we attempted to acquire detailed anatomy of the posterior eye.
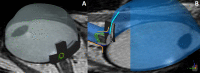
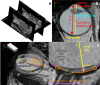
Methods: Optical and display distortions in OCT images were corrected using the Listing reduced eye model. The 3.0T orbital MRI images were placed on the three-dimensional coordinate system of the computer-aided design (CAD) program. Employing anterior scleral canal opening, visual axis, and scleral curvature as references, original and corrected OCT images were ported into the CAD application. The radii of curvature of the choroid-scleral interfaces (Rc values) of all original and corrected OCT images were compared to the MRI images.
Results: Sixty-five eyes of 33 participants (45.58 ± 19.82 years) with a mean Rc of 12.94 ± 1.24 mm on axial MRI and 13.66 ± 2.81 mm on sagittal MRI were included. The uncorrected horizontal OCT (30.51 ± 9.34 mm) and the uncorrected vertical OCT (34.35 ± 18.09 mm) lengths differed significantly from the MRI Rc values (both P < 0.001). However, the mean Rc values of the corrected horizontal (12.50 ± 1.21 mm) and vertical (13.05 ± 1.98 mm) images did not differ significantly from the Rc values of the corresponding MRI planes (P = 0.065 and P = 0.198, respectively).
Conclusions: Features identifiable only on OCT and features only on MRI were successfully integrated into a unitary posterior eye.
Translational relevance: Our CAD-based converging method may establish the collective anatomy of the posterior eye and the neural canal, beyond the range of the OCT.
Conflict of interest statement
Disclosure:
Figures






References
-
- Podoleanu A, Charalambous I, Plesea L, Dogariu A, Rosen R.. Correction of distortions in optical coherence tomography imaging of the eye. Phys Med Biol. 2004; 49: 1277–1294. - PubMed
-
- Berrio E, Tabernero J, Artal P.. Optical aberrations and alignment of the eye with age. J Vis. 2010; 10(14): 34. - PubMed
-
- Snead MP, Hardman Lea S, Rubinstein MP, Reynolds K, Haworth SM. Determination of the nodal point position in the pseudophakic eye. Ophthalmic Physiol Opt. 1991; 11: 105–108. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

