What lies on macroalgal surface: diversity of polysaccharide degraders in culturable epiphytic bacteria
- PMID: 35895126
- PMCID: PMC9329506
- DOI: 10.1186/s13568-022-01440-8
What lies on macroalgal surface: diversity of polysaccharide degraders in culturable epiphytic bacteria
Abstract
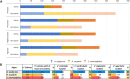
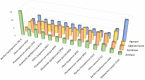
Macroalgal surface constitutes a peculiar ecological niche and an advantageous substratum for microorganisms able to degrade the wide diversity of algal glycans. The degrading enzymatic activities of macroalgal epiphytes are of paramount interest for the industrial by-product sector and biomass resource applications. We characterized the polysaccharide hydrolytic profile of bacterial isolates obtained from three macroalgal species: the red macroalgae Asparagopsis taxiformis and Sphaerococcus coronopifolius (Rhodophyceae) and the brown Halopteris scoparia (Phaeophyceae), sampled in South Portugal. Bacterial enrichment cultures supplemented with chlorinated aliphatic compounds, typically released by marine algae, were established using as inoculum the decaying biomass of the three macroalgae, obtaining a collection of 634 bacterial strains. Although collected from the same site and exposed to the same seawater seeding microbiota, macroalgal cultivable bacterial communities in terms of functional and phylogenetic diversity showed host specificity. Isolates were tested for the hydrolysis of starch, pectin, alginate and agar, exhibiting a different hydrolytic potential according to their host: A. taxiformis showed the highest percentage of active isolates (91%), followed by S. coronopifolius (54%) and H. scoparia (46%). Only 30% of the isolates were able to degrade starch, while the other polymers were degraded by 55-58% of the isolates. Interestingly, several isolates showed promiscuous capacities to hydrolyze more than one polysaccharide. The isolate functional fingerprint was statistically correlated to bacterial phylogeny, host species and enrichment medium. In conclusion, this work depicts macroalgae as holobionts with an associated microbiota of interest for blue biotechnologies, suggesting isolation strategies and bacterial targets for polysaccharidases' discovery.
Keywords: Bioprospecting; Blue biotechnology; Cultivable microbiota; Epibiota; Polysaccharidases; Seaweeds.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
The Cultivable Surface Microbiota of the Brown Alga Ascophyllum nodosum is Enriched in Macroalgal-Polysaccharide-Degrading Bacteria.Front Microbiol. 2015 Dec 24;6:1487. doi: 10.3389/fmicb.2015.01487. eCollection 2015. Front Microbiol. 2015. PMID: 26734000 Free PMC article.
-
Epiphytic common core bacteria in the microbiomes of co-located green (Ulva), brown (Saccharina) and red (Grateloupia, Gelidium) macroalgae.Microbiome. 2023 Jun 1;11(1):126. doi: 10.1186/s40168-023-01559-1. Microbiome. 2023. PMID: 37264413 Free PMC article.
-
Microbial Population Changes in Decaying Ascophyllum nodosum Result in Macroalgal-Polysaccharide-Degrading Bacteria with Potential Applicability in Enzyme-Assisted Extraction Technologies.Mar Drugs. 2019 Mar 29;17(4):200. doi: 10.3390/md17040200. Mar Drugs. 2019. PMID: 30934874 Free PMC article.
-
Microorganisms living on macroalgae: diversity, interactions, and biotechnological applications.Appl Microbiol Biotechnol. 2014 Apr;98(7):2917-35. doi: 10.1007/s00253-014-5557-2. Epub 2014 Feb 22. Appl Microbiol Biotechnol. 2014. PMID: 24562178 Review.
-
The seaweed holobiont: understanding seaweed-bacteria interactions.FEMS Microbiol Rev. 2013 May;37(3):462-76. doi: 10.1111/1574-6976.12011. Epub 2012 Dec 10. FEMS Microbiol Rev. 2013. PMID: 23157386 Review.
Cited by
-
A pan-genomic assessment: Delving into the genome of the marine epiphyte Bacillus altitudinis strain 19_A and other very close Bacillus strains from multiple environments.Heliyon. 2024 Mar 20;10(7):e27820. doi: 10.1016/j.heliyon.2024.e27820. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38560215 Free PMC article.
-
Physiological and intestinal microbiota responses of sea cucumber Apostichopus japonicus to various stress and signatures of intestinal microbiota dysbiosis.Front Microbiol. 2024 Dec 23;15:1528275. doi: 10.3389/fmicb.2024.1528275. eCollection 2024. Front Microbiol. 2024. PMID: 39780943 Free PMC article.
-
Rhodoalgimonas zhirmunskyi gen. nov., sp. nov., a Marine Alphaproteobacterium Isolated from the Pacific Red Alga Ahnfeltia tobuchiensis: Phenotypic Characterization and Pan-Genome Analysis.Microorganisms. 2023 Sep 30;11(10):2463. doi: 10.3390/microorganisms11102463. Microorganisms. 2023. PMID: 37894121 Free PMC article.
-
Exploring Diversity and Polymer Degrading Potential of Epiphytic Bacteria Isolated from Marine Macroalgae.Microorganisms. 2022 Dec 19;10(12):2513. doi: 10.3390/microorganisms10122513. Microorganisms. 2022. PMID: 36557766 Free PMC article.
References
-
- Anderson MJ, Gorley RN, Clarke KR. +for PRIMER: guide to software and statistical methods. Plymouth: PRIMER-E; 2008.

