Endoplasmic reticulum stress contributes to cisplatin-induced chronic kidney disease via the PERK-PKCδ pathway
- PMID: 35895146
- PMCID: PMC11072288
- DOI: 10.1007/s00018-022-04480-2
Endoplasmic reticulum stress contributes to cisplatin-induced chronic kidney disease via the PERK-PKCδ pathway
Abstract
Background: Cisplatin is an effective chemotherapeutic drug, but it may induce both acute and chronic kidney problems. The pathogenesis of chronic kidney disease (CKD) associated with cisplatin chemotherapy remains largely unclear.
Methods: Mice and renal tubular cells were subjected to repeated low-dose cisplatin (RLDC) treatment to induce CKD and related pathological changes. The roles of endoplasmic reticulum (ER) stress, PERK, and protein kinase C-δ (PKCδ) were determined using pharmacological inhibitors and genetic manipulation.
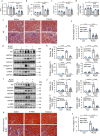
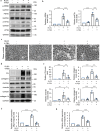
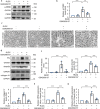
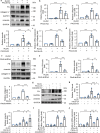
Results: ER stress was induced by RLDC in kidney tubular cells in both in vivo and in vitro models. ER stress inhibitors given immediately after RLDC attenuated kidney dysfunction, tubular atrophy, kidney fibrosis, and inflammation in mice. In cultured renal proximal tubular cells, inhibitors of ER stress or its signaling kinase PERK also suppressed RLDC-induced fibrotic changes and the expression of inflammatory cytokines. Interestingly, RLDC-induced PKCδ activation, which was blocked by ER stress or PERK inhibitors, suggesting PKCδ may act downstream of PERK. Indeed, suppression of PKCδ with a kinase-dead PKCδ (PKCδ-KD) or Pkcδ-shRNA attenuated RLDC-induced fibrotic and inflammatory changes. Moreover, the expression of active PKCδ-catalytic fragment (PKCδ-CF) diminished the beneficial effects of PERK inhibitor in RLDC-treated cells. Co-immunoprecipitation assay further suggested PERK binding to PKCδ.
Conclusion: These results indicate that ER stress contributes to chronic kidney pathologies following cisplatin chemotherapy via the PERK-PKCδ pathway.
Keywords: Chronic nephrotoxicity; Kidney repair; Protein kinase C-δ; Unfolded protein response.
© 2022. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
All authors declared no competing interests.
Figures



References
-
- Galgamuwa R, Hardy K, Dahlstrom JE, Blackburn AC, Wium E, Rooke M, Cappello JY, Tummala P, Patel HR, Chuah A, Tian L, McMorrow L, Board PG, Theodoratos A. Dichloroacetate prevents cisplatin-induced nephrotoxicity without compromising cisplatin anticancer properties. J Am Soc Nephrol. 2016;27(11):3331–3344. doi: 10.1681/ASN.2015070827. - DOI - PMC - PubMed
-
- Gu X, Yang H, Sheng X, Ko YA, Qiu C, Park J, Huang S, Kember R, Judy RL, Park J, Damrauer SM, Nadkarni G, Loos RJF, My VTH, Chaudhary K, Bottinger EP, Paranjpe I, Saha A, Brown C, Akilesh S, Hung AM, Palmer M, Baras A, Overton JD, Reid J, Ritchie M, Rader DJ, Susztak K. Kidney disease genetic risk variants alter lysosomal beta-mannosidase (MANBA) expression and disease severity. Sci Transl Med. 2021 doi: 10.1126/scitranslmed.aaz1458. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

