Experimental Rickettsia typhi Infection in Monodelphis domestica: Implications for Opossums as an Amplifying Host in the Suburban Cycle of Murine Typhus
- PMID: 35895366
- PMCID: PMC9294699
- DOI: 10.4269/ajtmh.21-1076
Experimental Rickettsia typhi Infection in Monodelphis domestica: Implications for Opossums as an Amplifying Host in the Suburban Cycle of Murine Typhus
Abstract
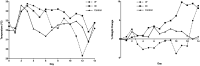
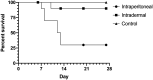
Murine typhus is an acute undifferentiated febrile illness caused by Rickettsia typhi. In the United States, its reemergence appears to be driven by a shift from the classic rat-rat flea cycle of transmission to one involving opossums (Didelphis virginiana) and cat fleas. Little is known of the ability of opossums to act as a reservoir and amplifying host for R. typhi. Here, we use Monodelphis domestica (the laboratory opossum) as a surrogate for D. virginiana. Opossums were inoculated via the intraperitoneal (IP) or intradermal (ID) route with 1 × 106 viable R. typhi. Blood and tissues were collected on days 6, 13, 20, and 27 or if moribund. Although one ID-infected opossum died, the remainder did not appear ill, whereas half of the IP-inoculated animals succumbed to infection. Rickettsemia was demonstrated in all animals through week 2 of infection and sporadically in weeks 3 and 4. Rickettsia typhi DNA was detected in all tissues, with most animals demonstrating the presence of bacteria into weeks 3 and 4. Histopathology and immunohistochemistry demonstrated typical findings of rickettsial infection. Akin to infection in rats, the demonstration of disseminated infection, typical inflammation, and prolonged rickettsemia with relatively few clinical effects (especially in the more natural route of ID inoculation) supports the potential of opossums to act as a competent mammalian reservoir and component of the zoonotic maintenance cycle of R. typhi. Understanding the dynamics of infection within opossums may have implications for the prevention and control of murine typhus.
Figures


References
-
- Yu X-J Walker DH , 2005. Family I. Rickettsiaceae. Brenner DJ, Kreig NR, Stanley JT, eds. Bergey’s Manual of Systematic Bacteriology. New York, NY: Springer, 96–116.
-
- Azad AF , 1990. Epidemiology of murine typhus. Annu Rev Entomol 35: 553–569. - PubMed
-
- Traub R Wisseman CL Farhang-Azad A , 1978. The ecology of murine typhus-a critical review. Trop Dis Bull 75: 237–317. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

