Host-Directed Targeting of LincRNA-MIR99AHG Suppresses Intracellular Growth of Mycobacterium tuberculosis
- PMID: 35895506
- PMCID: PMC7613730
- DOI: 10.1089/nat.2022.0009
Host-Directed Targeting of LincRNA-MIR99AHG Suppresses Intracellular Growth of Mycobacterium tuberculosis
Abstract
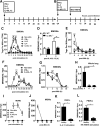
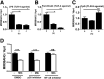
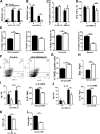
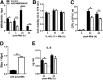
Tuberculosis (TB) caused by Mycobacterium tuberculosis (Mtb) kills 1.6 million people worldwide every year, and there is an urgent need for targeting host-pathogen interactions as a strategy to reduce mycobacterial resistance to current antimicrobials. Noncoding RNAs are emerging as important regulators of numerous biological processes and avenues for exploitation in host-directed therapeutics. Although long noncoding RNAs (lncRNAs) are abundantly expressed in immune cells, their functional role in gene regulation and bacterial infections remains understudied. In this study, we identify an immunoregulatory long intergenic noncoding RNA, lincRNA-MIR99AHG, which is upregulated in mouse and human macrophages upon IL-4/IL-13 stimulation and downregulated after clinical Mtb HN878 strain infection and in peripheral blood mononuclear cells from active TB patients. To evaluate the functional role of lincRNA-MIR99AHG, we used antisense locked nucleic acid (LNA) GapmeR-mediated antisense oligonucleotide (ASO) lncRNA knockdown experiments. Knockdown of lincRNA-MIR99AHG with ASOs significantly reduced intracellular Mtb growth in mouse and human macrophages and reduced pro-inflammatory cytokine production. In addition, in vivo treatment of mice with MIR99AHG ASOs reduced the mycobacterial burden in the lung and spleen. Furthermore, in macrophages, lincRNA-MIR99AHG is translocated to the nucleus and interacts with high affinity to hnRNPA2/B1 following IL-4/IL-13 stimulation and Mtb HN878 infection. Together, these findings identify lincRNA-MIR99AHG as a positive regulator of inflammation and macrophage polarization to promote Mtb growth and a possible target for adjunctive host-directed therapy against TB.
Keywords: M. tuberculosis; host-directed therapy; inflammation; long noncoding RNAs; macrophages.
Conflict of interest statement
No competing financial interests exist.
Figures

Similar articles
-
Differential Targeting of c-Maf, Bach-1, and Elmo-1 by microRNA-143 and microRNA-365 Promotes the Intracellular Growth of Mycobacterium tuberculosis in Alternatively IL-4/IL-13 Activated Macrophages.Front Immunol. 2019 Mar 19;10:421. doi: 10.3389/fimmu.2019.00421. eCollection 2019. Front Immunol. 2019. PMID: 30941122 Free PMC article.
-
Triptolide Modulates the Expression of Inflammation-Associated lncRNA-PACER and lincRNA-p21 in Mycobacterium tuberculosis-Infected Monocyte-Derived Macrophages.Front Pharmacol. 2021 Apr 12;12:618462. doi: 10.3389/fphar.2021.618462. eCollection 2021. Front Pharmacol. 2021. PMID: 33912039 Free PMC article.
-
Transcriptional landscape of Mycobacterium tuberculosis infection in macrophages.Sci Rep. 2018 Apr 30;8(1):6758. doi: 10.1038/s41598-018-24509-6. Sci Rep. 2018. PMID: 29712924 Free PMC article.
-
The Role of microRNAs and Long Non-Coding RNAs in the Regulation of the Immune Response to Mycobacterium tuberculosis Infection.Front Immunol. 2021 Jun 24;12:687962. doi: 10.3389/fimmu.2021.687962. eCollection 2021. Front Immunol. 2021. PMID: 34248974 Free PMC article. Review.
-
[Development of antituberculous drugs: current status and future prospects].Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
Cited by
-
Unveiling the Hidden Regulators: The Impact of lncRNAs on Zoonoses.Int J Mol Sci. 2024 Mar 21;25(6):3539. doi: 10.3390/ijms25063539. Int J Mol Sci. 2024. PMID: 38542512 Free PMC article. Review.
-
Antisense Therapy for Infectious Diseases.Cells. 2023 Aug 21;12(16):2119. doi: 10.3390/cells12162119. Cells. 2023. PMID: 37626929 Free PMC article. Review.
-
Long non-coding RNA transcripts in Mycobacterium tuberculosis-host interactions.Noncoding RNA Res. 2024 Dec 15;11:281-293. doi: 10.1016/j.ncrna.2024.12.005. eCollection 2025 Apr. Noncoding RNA Res. 2024. PMID: 39926616 Free PMC article. Review.
-
Exploring cellular changes in ruptured human quadriceps tendons at single-cell resolution.J Physiol. 2025 Aug;603(16):4535-4554. doi: 10.1113/JP287812. Epub 2025 Apr 15. J Physiol. 2025. PMID: 40232153 Free PMC article.
-
LncRNA MIR99AHG serves as a diagnostic marker for early lung cancer and regulates cancer cell growth and metastasis via miR-194-5p.Discov Oncol. 2025 Jun 10;16(1):1050. doi: 10.1007/s12672-025-02896-3. Discov Oncol. 2025. PMID: 40495005 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

