Casirivimab and Imdevimab for the Treatment of Hospitalized Patients With COVID-19
- PMID: 35895508
- PMCID: PMC9384575
- DOI: 10.1093/infdis/jiac320
Casirivimab and Imdevimab for the Treatment of Hospitalized Patients With COVID-19
Abstract
Background: The open-label RECOVERY study reported improved survival in hospitalized, SARS-CoV-2 seronegative patients treated with casirivimab and imdevimab (CAS + IMD).
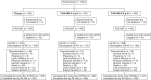
Methods: In this phase 1/2/3, double-blind, placebo-controlled trial conducted prior to widespread circulation of Delta and Omicron, hospitalized COVID-19 patients were randomized (1:1:1) to 2.4 g or 8.0 g CAS + IMD or placebo, and characterized at baseline for viral load and SARS-CoV-2 serostatus.
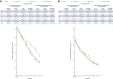
Results: In total, 1336 patients on low-flow or no supplemental (low-flow/no) oxygen were treated. The primary endpoint was met in seronegative patients, the least-squares mean difference (CAS + IMD versus placebo) for time-weighted average change from baseline in viral load through day 7 was -0.28 log10 copies/mL (95% confidence interval [CI], -.51 to -.05; P = .0172). The primary clinical analysis of death or mechanical ventilation from day 6 to 29 in patients with high viral load had a strong positive trend but did not reach significance. CAS + IMD numerically reduced all-cause mortality in seronegative patients through day 29 (relative risk reduction, 55.6%; 95% CI, 24.2%-74.0%). No safety concerns were noted.
Conclusions: In hospitalized COVID-19 patients on low-flow/no oxygen, CAS + IMD reduced viral load and likely improves clinical outcomes in the overall population, with the benefit driven by seronegative patients, and no harm observed in seropositive patients.
Clinical trials registration: NCT04426695.
Keywords: COVID-19; SARS-CoV-2; coronavirus; hospitalized; monoclonal antibody.
Plain language summary
Lay Summary . Monoclonal antibody therapies that block the virus that causes COVID-19 (SARS-CoV-2) can prevent patients from being hospitalized. We hypothesized that these antibodies may also benefit patients who are already hospitalized with COVID-19. Therefore, we performed a study to determine if the monoclonal antibody combination of casirivimab and imdevimab (CAS + IMD) can decrease the amount of virus in the nose of hospitalized patients and prevent the disease from becoming more severe. The study, conducted from June 2020 to April 2021, found that CAS + IMD treatment reduced the amount of virus in these patients, and may reduce their chance of dying or needing a ventilator (a machine that helps patients breathe). Patients were examined in 2 groups: those whose immune systems, at the start of the study, had not produced their own antibodies to fight SARS-CoV-2 (seronegative patients); or those that had already produced their own antibodies (seropositive patients) at the start of the study. Seronegative patients benefited the most from CAS + IMD. No safety concerns related to CAS + IMD were observed. These results demonstrate that monoclonal antibody therapy can help hospitalized patients with COVID-19 and may decrease their chances of needing assistance to breathe or dying.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. S. S.-K., S. A., Y. Sun, R. B., J. Mei, J. Miller, E. F.-N., C. P., V. P., Y. Z., A. M., J. D. D., Y. K., A. C., B. K., Y. Soo, A. T. D., G. P. G., L. L., N. B., and D. M. W. are employees/stockholders of Regeneron Pharmaceuticals, Inc, and report grants from Biomedical Advanced Research and Development Authority (BARDA). E. M. reports payments to his institution received from National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases, NIH/National Institute of General Medical Sciences, SciClone Pharmaceuticals, Regeneron Pharmaceuticals, Inc, Pfizer, Chemic Labs/KODA Therapeutics, Cidara, and Leidos Biomedical Research Inc/NCI. V. P. M. and J. C. W. report grants from BARDA. S. S. is an Excision BioTherapeutics employee/stockholder and former Regeneron Pharmaceuticals, Inc, employee and current stockholder, and reports grants from BARDA. L. C. is a Regeneron Pharmaceuticals, Inc employee and reports grants from BARDA. A. T. H. is a Regeneron Pharmaceuticals, Inc employee/stockholder, a former Pfizer employee and current stockholder, has a patent pending with Regeneron Pharmaceuticals, Inc and reports grants from BARDA. J. D. H., K. C. T., and G. A. H. are employees/stockholders of Regeneron Pharmaceuticals, Inc and have a patent pending, which has been licensed and receiving royalties, with Regeneron Pharmaceuticals, Inc. R. H. is a former employee and current stockholder of Regeneron Pharmaceuticals, Inc, and reports grants from BARDA. N. S. and G. D. Y. are employees/stockholders of Regeneron Pharmaceuticals, Inc, and have issued patents (US Patent Nos. 10 787 501, 10 954 289, and 10 975 139) and pending patents, which have been licensed and receiving royalties, with Regeneron Pharmaceuticals, Inc, and reports grants from BARDA. Funding to pay the Open Access publication charges for this article was provided by Regeneron Pharmaceuticals, Inc.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

