Longitudinal monitoring of SARS-CoV-2 spike protein-specific antibody responses in Lower Austria
- PMID: 35895668
- PMCID: PMC9328535
- DOI: 10.1371/journal.pone.0271382
Longitudinal monitoring of SARS-CoV-2 spike protein-specific antibody responses in Lower Austria
Abstract
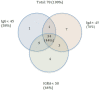
The Lower Austrian Wachau region was an early COVID-19 hotspot of infection. As previously reported, in June 2020, after the first peak of infections, we determined that 8.5% and 9.0% of the participants in Weißenkirchen and surrounding communities in the Wachau region were positive for immunoglobulin G (IgG) and immunoglobulin A (IgA) antibodies against the receptor-binding domain of the spike protein of SARS-CoV-2, respectively. Here, we present novel data obtained eight months later (February 2021) from Weißenkirchen, after the second peak of infection, with 25.0% (138/552) and 23.6% (130/552) of participants that are positive for IgG and IgA, respectively. In participants with previous IgG/IgA positivity (June 2020), we observed a 24% reduction in IgG levels, whereas the IgA levels remained stable in February 2021. This subgroup was further analyzed for SARS-CoV-2 induced T cell activities. Although 76% (34/45) and 76% (34/45) of IgG positive and IgA positive participants, respectively, showed specific T cell activities (upon exposure to SARS-CoV-2 spike protein-derived peptides), those were not significantly correlated with the levels of IgG or IgA. Thus, the analyses of antibodies cannot surrogate the measurement of T cell activities. For a comprehensive view on SARS-CoV-2-triggered immune responses, the measurement of different classes of antibodies should be complemented with the determination of T cell activities.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

