Investigating microscale patchiness of motile microbes under turbulence in a simulated convective mixed layer
- PMID: 35895753
- PMCID: PMC9380958
- DOI: 10.1371/journal.pcbi.1010291
Investigating microscale patchiness of motile microbes under turbulence in a simulated convective mixed layer
Abstract
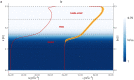
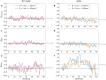
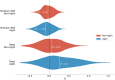
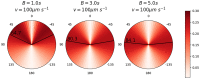
Microbes play a primary role in aquatic ecosystems and biogeochemical cycles. Spatial patchiness is a critical factor underlying these activities, influencing biological productivity, nutrient cycling and dynamics across trophic levels. Incorporating spatial dynamics into microbial models is a long-standing challenge, particularly where small-scale turbulence is involved. Here, we combine a fully 3D direct numerical simulation of convective mixed layer turbulence, with an individual-based microbial model to test the key hypothesis that the coupling of gyrotactic motility and turbulence drives intense microscale patchiness. The fluid model simulates turbulent convection caused by heat loss through the fluid surface, for example during the night, during autumnal or winter cooling or during a cold-air outbreak. We find that under such conditions, turbulence-driven patchiness is depth-structured and requires high motility: Near the fluid surface, intense convective turbulence overpowers motility, homogenising motile and non-motile microbes approximately equally. At greater depth, in conditions analogous to a thermocline, highly motile microbes can be over twice as patch-concentrated as non-motile microbes, and can substantially amplify their swimming velocity by efficiently exploiting fast-moving packets of fluid. Our results substantiate the predictions of earlier studies, and demonstrate that turbulence-driven patchiness is not a ubiquitous consequence of motility but rather a delicate balance of motility and turbulent intensity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Turbulence drives microscale patches of motile phytoplankton.Nat Commun. 2013;4:2148. doi: 10.1038/ncomms3148. Nat Commun. 2013. PMID: 23852011
-
Emergence of phytoplankton patchiness at small scales in mild turbulence.Proc Natl Acad Sci U S A. 2018 Nov 27;115(48):12112-12117. doi: 10.1073/pnas.1808711115. Epub 2018 Nov 8. Proc Natl Acad Sci U S A. 2018. PMID: 30409800 Free PMC article.
-
Turbulence induces clustering and segregation of non-motile, buoyancy-regulating phytoplankton.J R Soc Interface. 2019 Oct 31;16(159):20190324. doi: 10.1098/rsif.2019.0324. Epub 2019 Oct 23. J R Soc Interface. 2019. PMID: 31640498 Free PMC article.
-
Gyrotactic phytoplankton in laminar and turbulent flows: A dynamical systems approach.Eur Phys J E Soft Matter. 2019 Mar 20;42(3):31. doi: 10.1140/epje/i2019-11792-0. Eur Phys J E Soft Matter. 2019. PMID: 30879226 Review.
-
Turbulence as a framework for brain dynamics in health and disease.Neurosci Biobehav Rev. 2025 Feb;169:105988. doi: 10.1016/j.neubiorev.2024.105988. Epub 2024 Dec 22. Neurosci Biobehav Rev. 2025. PMID: 39716558 Review.
References
-
- Azam F, Fenchel T, Field J, Gray J, Meyer-Reil L, Thingstad F. The Ecological Role of Water-Column Microbes in the Sea. Marine Ecology Progress Series. 1983;10:257–263. doi: 10.3354/meps010257 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

